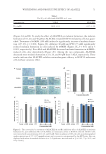
SURFACTANT PENETRATION 99 skin lipid matrix, Lémery et al. (35) studied this at three different levels: (i) the inves- tigation of the skin polarity change which is the key to the skin wetting by surfactants. The increase in the skin polarity implies that the surfactants tend to remain on the skin surface (ii) using infrared spectroscopy in the attenuated total refl ectance mode to char- acterize the disorganization extent of the lipid matrix induced by different surfactants. The shift of the stretching peak from 2,849.8 to 2,850.5 cm−1 indicates the phase change from the OR to the HEX, and the shift from 2,850.7 to 2,854.2 cm−1 suggests the HEX phase changes to the liquid crystalline state (36). As the fl uidity of the lipid structure increases, the disorganization of the lipid matrix increases as well and (iii) quantitative analysis of the change in the SC lipids by using High Performance Liquid Chromatogra- phy (HPLC). The HPLC characterization revealed the amount of total extracted skin lipids as well as the relative changes in the various lipids, i.e., squalene, TS, CERs, and CHOL (35,37–39). They are the four model compounds representing the main skin se- bum and lipid components. Motta S et al. found out that when there was damage in the skin barrier, the CER contents presented a decreasing trend (40). CHOL is the most im- portant sterol in the SC. When its synthesis is impeded or its content decreases, the skin barrier dysfunction occurs (41). Squalene in sebum can effectively block the chain reac- tion of free radicals and inhibit sebum peroxidation, thereby protecting the skin from external stimulation. TS play a vital role in skin injury repair. Consequently, the contents of the squalene and TS are also important to judge skin conditions (42,43). Any variation in the structure, content, and proportion of these four lipid components can impact the skin barrier functions. In the study conducted by Lémery et al. (35), they chose 10 com- mon commercial surfactants (as shown in Figure 6) from different categories: two anionic surfactants (sodium stearoyl lactylate and SLS), two cationic surfactants (cetyltrimethyl- ammonium chloride and distearyldimonium chloride), and six nonionic surfactants (PEG-100 stearate, laureth-23, PEG-12 dimethicone, hydrogenated lecithin, PEG-25 hydrogenated castor oil, and polyoxyethylene sorbitan laurate). The corresponding results are summarized in Table III (35). Lemery et al. (35) demonstrated that SLS, and cetyl trimethylammonium chloride (CTAC) were considered to be the most irritating surfactants. They remained on the skin surface, disorganized the SC lipid matrix, and extracted most of the skin lipids. On the other hand, the PEG-12 dimethicone and the polyoxyethylene sorbitan laurate had no obvious effects on the skin barrier compared with the rest surfactants (35). They did not induce a shift in the skin polarity or a change in the lipid crystalline structures. In addition, these two nonionic surfactants did not dissolve or remove any signifi cant amount of the skin lipids. These two mild surfactants both possess large PEG head groups, suggesting that they are hindered from the skin barrier (44). THE IMPACT OF SURFACTANTS TO PROTEINS Proteins belong to biomacromolecules. Different amino acids are polymerized and folded into a three-dimensional structure (45,46). Proteins are copolymers possessing both hy- drophilic and hydrophobic parts, in which the hydrophilic groups can be both ionic or nonionic (47). In general, the interactions between surfactants and proteins include two stages: nonoperative binding and co-operative binding (1). The nonoperative binding is also named site-specifi c binding. The surfactant monomers in this stage attach to the specifi c sites of proteins. This binding occurs especially in the presence of ionic surfactants,
JOURNAL OF COSMETIC SCIENCE 100 Figure 6. Ten common commercial surfactants used in the study conducted by Lémery et al. (35).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)