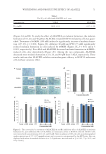
WHITENING AND PROTECTIVE EFFECT OF ALACELL 73 cells at four concentrations (Figure 3A). However, cells treated with ALACELL for 24 hr at concentrations of 80 and 100 μM before the UV irradiation were signifi cantly pro- tected against the loss of viability (Figure 3A and p 0.05). Also, the survival of HaCaT cells exposed to UVB (80 mJ/cm2) for 24 h decreased signifi cantly to 62.56% (p 0.001), and ALACELL concentrations of 80 and 100 μM for 24 h signifi cantly increased cell viability (Figure 3B, p 0.05 and p 0.01, respectively). The UVB-irradiated (120 mJ/cm2) HaCaT cells showed cell viability of 45.78% (Figure 3C and p 0.001), but ALACELL treated with four concentrations signifi cantly recovered cell viability (Figure 3C and p 0.01 for four concentrations). DISCUSSION Acne is a type of chronic infl ammatory disease that gen eral appear s as pustules (pimples) and nodules on human skin (24). Furthermore, acne can generate psychological and social issues, and have a grave effect on the quality of human life (15). Therefore, many research- ers are focused on detecting safe and effective ways to treat acne. A variety of treatments are available for acne, including antibact erial agents, natural com- pounds, and hormone therapy. However, these treatments have limitations in the clinic, where, for example, retinoids have several side effects and antibiotics may cause resistance (25). PDT has played an important role in dermatological treatment and o ffers alternatives to people who perform topical treatments. Treating 5-ALA-PDT, 5-ALA is taken in by epi- thelial cells and synthesized into protoporphyrin IX through the biosynthetic pathway, and then photoactivated porphyrin is formed from the making of singlet oxygen and other potent oxidizers that produce antimicrobial and anti-infl ammatory effi cacy (16). In this study, ALACELL showed growth inhibitory effect against C. acnes, but there was no inhibitory effect against other strains (S. aureus, B. cereus, E. coli, and Y. enterocolitica). In addition, the inhibitory effect of ALACELL on C. acnes was higher than that of 5-ALA. Skin aging is a biochemical process caused by many individual factors, such as UV light exposure (26). Various factors cause skin aging, including wrinkles and pigmentation (27). In this study, to look for other effects of ALACELL on human skin as well as its inhibitory effect on C. acnes, melanin formation and tyrosinase activity from the treat- ment of ALACELL were measured in B16F10 melanoma cells. As a result, ALACELL sig- nifi cantly decreased the α-MSH–induced melanin formation and tyrosinase activity in B16F10 cells dose dependently, and ALACELL was higher than 5-ALA. Therefore, this study found that 5-ALA–based synthesized ALACELL effi ciently showed whitening effects, resulting in the decrease of α-MSH–induced melanin formation and tyrosinase activity in B16F10 cells. Continuous UVB irradiation permeates human skin and produces intracellu lar ROS, re- sulting in various skin changes, including rough skin, dryness, and wrinkle formation (28). Consumers are increasingly looking to various ingredients to improve the appearance of their skin and to delay the effects of aging. This study evaluated the protective effects of ALACELL on UVB-irradiated cell death by irradiation of 40, 80, and 120 mJ/cm2. ALACELL concentration of 80 and 100 μM signifi cantly increased cell viability at irradiations of 40, 80, and 120 mJ/cm2 for 24 h. Its protective effi cacy was higher than that of 5-ALA. From these results, it is concluded that the 5-ALA–based synthesized ALACELL may be a pho- toprotection candidate for skin care.
JOURNAL OF COSMETIC SCIENCE 74 ACKNOWLEDGMENTS We would like to thank Kyung-Chan Kim at Unique Medicare Co., Ltd. and Dae-Hyen Jung at BIO-FD&C. REFERENCES (1) J. Liu, R. Yan, Q. Zhong, S. Ngo, N. J. Bangayan, L. Nguyen, T. Lui, M. Liu, M. C. Erfe, N. Craft, S. Tomida, and H. Li, The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin, ISME J., 9(9), 2078–2093 (2015). (2) E. A. Grice, The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutane- ous disease, Semin. Cutan. Med. Surg., 33(2), 98–103 (2014). (3) E. Jonczyk-Matysiak, B. Weber-Dabrowska, M. Zaczek, R. Międzybrodzki, S. Letkiewicz, M. Lusiak- Szelchowska, and A. Górski, Prospects of phage application in the treatment of acne caused by Propioni- bacterium acnes, Front. Microbiol., 8, 164 (2017). (4) P. F. Liu, Y. D. Hsieh, Y. C. Li n , A. Two, C. W. Shu, and C. M. Huang, Propionibacterium acnes in the pathogenesis and immunotherapy of acne vulgaris, Curr. Drug Metabol., 16(4), 245–254 (2015). (5) J. Kim, M. T. Ochoa, S. R. Krutz i k, O. Takeuchi, S. Uematsu, A. J. Legaspi, H. D. Brightbill, D. Holland, W. J. Cunliffe, S. Akira, P. A. Sieling, and P. J. Godowski, Modlin RL activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses, J. Immunol., 169(3), 1535–1541 (2002). (6) S. Fitz-Gibbon, S. Tomida, B. H. C h iu, L. Nguyen, C. Du, M. Liu, D. Elashoff, M. C. Erfe, A. Loncaric, J. Kim, R. L. Modlin, J. F. Miller, E. Sodergren, N. Craft, G. M. Weinstock, and H. Li, Propionibacterium acnes strain populations in the human skin microbiome associated with acne, J. Invest. Dermatol., 133(9), 2152–2160 (2013). (7) H. Nasri, M. Bahmani, N. Shahinfar d , A. M. Nafchi, S. Saberianpour, and M. R. Kopaei, Medicinal plants for the treatment of acne vulgaris: a review of recent evidences, Jundishapur J. Microbiol., 8, e25580 (2015). (8) P. Coates, S. Vyakrnam, E. A. Eady , C. E. Jones, J. H. Cove, and W. J. Cunliffe, Prevalence of antibiotic- resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribu- tion study, Br. J. Dermatol., 146(5), 840–848 (2002). (9) S. A. D’Mello, G. J. Finlay, B. C. Baguley, and M. E. Askarian-Amiri, Signaling pathways in melano- genesis, Int. J. Mol. Sci., 17(7), E1144 (2016). (10) Pillaiyar T., Manickam M., and Ju n g S. H., Recent development of signaling pathways inhibitors of melanogenesis, Cell. Signal., 40, 99–115 (2017). (11) M. C. Cheng, T. H. Lee, Y. T. Chu , L. L. Syu, S. J. Hsu, C. H. Cheng, J. Wu, and C. K. Lee, Melanogen- esis inhibitors from the rhizoma of Ligusticum sinense in B16-F10 melanoma cells in vitro and zebrafi sh in vivo, Int. J. Mol. Sci., 19(12), E3994 (2018). (12) W. Zhu and J. Gao, The use of bot a nical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders, J. Invest. Dermatol. Symp. Proc., 13(1), 20–24 (2008). (13) Z. Kang, J. Zhang, J. Zhou, Q. Qi , G. Du, and J. Chen, Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12, Biotechnol. Adv., 30(6), 1533–1542 (2012). (14) M. Elman, M. Slatkine, and Y. Harth, The effective treatment of acne vulgaris by a high-int e nsity, nar- row band 405–420 nm light source, J. Cosmet. Laser Ther., 5(2), 111–117 (2003). (15) X. Chen, H. Song, S. Chen, J. Zhang, G. Niu, and X. Liu, Clinical effi cacy of 5-aminolevulinic acid photodynamic therapy in the treatment of moderate to severe facial acne vulgaris, Exp. Th e r. Med., 10(3), 1194–1198 (2015). (16) B. Pollock, D. Turner, M. R. Stringer, R. A. Bojar, V. Goulden, G. I. Stables, and W. J. Cunliffe, Topical aminolaevulinic acid-photodynamic therapy for the treatment of acne vulgaris: a stu d y of clinical effi - cacy and mechanism of action, Br. J. Dermatol., 151(3), 616–622 (2004). (17) M. S. Nestor, M. H. Gold, A. N. Kauvar, A. F. Taub, R. G. Geronemus, E. C. Ritvo, M. P. Goldman, D. J. Gilbert, D. F. Richey, T. S. Alster, R. R. Anderson, D. E. Bank, A. Carruthers, J. Carru t hers, D. J. Goldberg, C. W. Hanke, N. J. Lowe, D. M. Pariser, D. S. Rigel, P. Robins, J. M. Spencer, and B. D. Zelickson, The use of photodynamic therapy in dermatology: results of a consensus conference, J. Drugs Dermatol., 5(2), 140–154 (2006). (18) A. Klein, P. Babilas, S. Karrer, M. Landthaler, and R. M. Szeimies, Photodynamic therapy in dermatol- ogy - an update 2008, J. Dtsch. Dermatol. Ges., 6(10), 839–846 (2008).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)