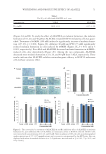
SURFACTANT PENETRATION 103 Clearly, contradicted behaviors were observed from the penetration experiments of SCI and SDS. Consequently, researchers did a series of studies and proposed hypotheses to perfect the surfactant penetration model. The CMC of SCI is indeed lower than that of SDS, inducing a smaller amount of monomers existing in the solution and the formation of micelles at a lower concentration (51). According to the monomer penetration model, a surfactant with a lower CMC is unlikely to cause high skin penetration and induce seri- ous irritation (54), which partially explains why SCI is milder than SDS (55). But how about the penetration behaviors of SCI micelles? To answer this question, Ghosh and Blankschtein (51) measured the radius of SCI micelles and the average aqueous pore ra- dius resulting from skin exposure to SCI by using dynamic light-scattering. The results as well as their comparison to SDS and control are presented in Table IV. Apparently, SCI micelles are larger than SDS micelles, which were also larger than the aver- age aqueous pore radius. This indicated that SCI micelles face steric hindrance to penetrate into the skin barrier. On the other hand, SDS micelles are small enough to be able to enter the skin aqueous pores. Therefore, micelle size is an important factor to determine the sur- factant penetration behaviors. Moreover, SDS is capable of increasing both the aqueous pore size and the number density thus, it could alter the skin structure, inducing skin irritation (51). Ghosh and Blankschtein (51) demonstrated that SCI only slightly induced skin per- turbation, thanks to its large micelle size and its ability to reduce aqueous pore radius/ density. As a result, SCI is a good candidate to be applied to mild cleansing. Hill et al. (56) and James-Smith et al. (57,58) proposed the submicelle penetration model. This hypothesis was based on micelle kinetics—micelles are rapidly breaking and reform- ing continuously (57–59). This dynamic state is described in Figure 7. The dynamic state includes two types of relevant time scales: fast relaxation time and slow relaxation time. Fast relaxation time is used to measure the time needed for the surfactants to enter or come out of the micelles. Slow relaxation time describes the time used for the micelles to completely form or completely integrate. In this equilibrium, some monomers could form aggregations smaller than micelles termed submicelles or premicelles. James-Smith et al. (58) observed SDS submicelles were presented at the concentration of 3–4 times CMC. Later, LeBard et al. (59) used the molecular dynamic simulations to investigate the dynamic change of a nonionic polyethylene glycol (PEG) surfactant, C7E6 solution, at low concentrations. Pre- micelles were identifi ed at concentrations below the CMC, and their concentration increased with increasing total concentration below the CMC, reaching a plateau above the CMC, where these premicelles exist in equilibrium with free monomers and full-size micelles (59). Because of the smaller sizes, the submicelles may have the ability to penetrate into the skin barrier (56–58). To verify this hypothesis, Hill et al. (57) adjusted the SDS micelle stability by mixing with dodecyl trimethylammonium bromide (C12TAB) at various ratios and ob- served that addition of C12TAB lowered the ability of SDS to perturb skin barrier properties Table IV Micelle Radius and the Average Aqueous Pore Radius after Skin Exposure to SCI/SDS/Phosphate-buffered saline (PBS) Control Solutions Types of solution Micelle radius, r (A) Average aqueous pore radius, rpore (A) Aqueous pore number density, (ε/τ)normal SCI solution 33.5 ± 1 29 ± 5 2 ± 1 SDS solution 19.5 ± 1 33 ± 5 7 ± 1 PBS control solution Not applicable 20 ± 3 1
JOURNAL OF COSMETIC SCIENCE 104 by decreasing the concentration of SDS monomers and submicelles. This indicates submi- celles indeed possess the ability to penetrate into the skin. A NEW PROPOSED MODEL: SURFACTANT CHARGE DENSITY AND PENETRATION CORRELATION To fi nd out which model is the most sophisticated to explain the surfactant penetration, Morris et al. (60) well studied the physicochemical parameters of surfactants that could infl uence their skin penetration: (i) the CMC, representing the amount of surfactant monomers in the solution (ii) the micelle radius, showing the steric hindrance of mi- celles and (iii) the zeta potential, correlating with the micelle charge density. Morris et al. (60) used the radiolabeled SDS (14C-SDS) to investigate 16 different surfactant systems in vitro on the human cadaver skin. The 16 surfactant systems and their physico- chemical parameters are listed in Table V. The study indicated that only (i) the CMC and (iii) the zeta potential showed a clear correlation with the radiolabeled SDS penetration. Interest- ingly, the SDS micelle radius did not show a clear correlation, which was inconsistent with the previous fi ndings. Morris et al. (60) hypothesized this was attributed to the short residence time of surfactants contacting the skin in the experiment. When the skin barrier is exposed to surfactants for a longer period, the micelle size would have an impact on the surfactant pene- tration. When anionic surfactant solution comes into contact with the skin, monomer pene- trates the skin and binds to proteins, increasing the electrical charge on the protein network and causing the skin structure to swell. This allows for progressive surfactant binding in deeper layers of the skin, resulting in enhanced skin swelling (57,61). Therefore, surfactant micelles and submicelles may additionally be able to penetrate and swell the skin structure. The more charged the surfactant system and the longer the surfactant exposure time, the more the binding to the skin proteins, speeding up the penetration process (33,34,57). Zeta poten- tial is known to correlate with the charge density of colloids in solution, and that is the reason why it was revealed to correlate with the surfactant penetration in the study. METHODS TO REDUCE SURFACTANT PENETRATION INTO SKIN All the hypotheses with respect to surfactant penetration discussed earlier suggest that the surfactant penetration is related to the steric interaction between the surfactant monomers/submicelles/micelles and the average aqueous pore radius/number density. Consequently, increasing surfactant monomer/micelle size and/or reducing average aqueous Figure 7. The dynamic state of surfactants at a certain concentration of surfactants (Reprinted from (58) with permission. Copyright 2007 Elsevier).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)