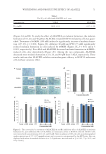
SURFACTANT PENETRATION 101 but this interaction is not free energetically favorable in the case of nonionic surfactants. In the second co-operative binding stage, the surfactants aggregate to form micellar structures interacting with proteins, destroying their secondary structures. In this case, unfolding of the spiral structures of proteins would occur, and the biological activities would also be lost (4). If the proteins in skin are denaturized by surfactants, they would be solubilized in the surfactant solution, and the skin would also swell (4). The behaviors of mixed surfactants dissolving proteins were studied by Moore et al. (48). In detail, they investigated how surfactants—SDS/C12 En (n = 4, 6, and 8) mixtures— dissolved the zein protein. Zein is a mixture of proteins with an average molecular weight from 25,000 to 45,000, and thus it is insoluble in water. However, surfactants can promote the zein aqueous solubility, and this solubility is correlated with the physico- chemical properties of surfactants. The solubilization of zein by surfactants is analogous to the interactions of surfactants with skin proteins (49). In addition, the skin swelling due to surfactants interacting with skin proteins can further promote the penetration of surfac- tants and other ingredients in the cleansing products, inducing skin irritation (4,33,48). As a result, the zein protein is a good material to evaluate skin mildness of surfactants. Demonstrated by Moore et al. (48), SDS indeed promoted the dissolution of the zein protein. Three possible reasons for the favorite interaction between the zein protein and SDS were proposed: (i) the protein backbone protects the hydrophobic patches which expose at the outside of surfactant micelles from the external aqueous environment, (ii) the hydrophobic side chains of the zein protein have the ability to enter the internal hy- drophobic core of micelles, and (iii) the interaction between the positively charged pro- tein and the negatively charged SDS contributes to the dissolution of the zein protein. Moreover, nonionic surfactants or certain amphoteric surfactants seldom cause serious zein dissolution compared with anionic and cationic surfactants (48). The polar heads of nonionic surfactants or certain amphoteric surfactants reduce the micelle charge density, Table III The Effect of 10 Surfactants on the Skin barrier. The “+” Marks an Evident Effect of Surfactants Polarity of skin Lipid matrix disorganization of skin Lipid extraction of skin SLS + + Total+ SSL + TS+ CHOL+ DC + + TS+ CERs+ CHOL+ CTAC + Total+ Laureth-23 + + CHOL+ Polyoxyethylene sorbitan laurate CERs+ CHOL+ PEG-100 stearate + Squalene+ CHOL+ PEG-12 dimethicone CHOL+ PEG-25 hydrogenated castor oil + + CERs+ CHOL+ Hydrogenated lecithin + + CERs+ CHOL+
JOURNAL OF COSMETIC SCIENCE 102 which induces the electrostatic interaction between the zein protein and SDS. In addi- tion, the sizes of polar heads of nonionic surfactants or certain amphoteric surfactants could increase the steric hindrance, thereby reducing the access of the hydrophobic side chains of proteins into internal micelles and the exposure of hydrophobic parts of surfac- tant micelles to the external environment (48). THE PENETRATION OF SURFACTANTS INTO SKIN As discussed earlier, one reason to cause the surfactant-induced skin irritation is the con- tact between surfactants and the lipids/proteins in the skin barrier. If the surfactant pen- etration into the skin can be inhibited, its contact with skin lipids/proteins can be reduced, so is the surfactant-induced skin irritation. To achieve this, the understanding in the mechanism of the surfactant penetration into the skin is essential. In the past two decades, many scientists have researched this topic. To summarize, there has been mainly three hypotheses respecting the mechanism of surfactant penetration into the skin. THE SURFACTANT MONOMER PENETRATION MODEL A widely accepted view of the surfactant penetration is termed as the monomer penetration model. This model explains that surfactant monomers can access the pathways through the skin barrier because they possess relatively small sizes. When the surfactant monomers penetrate into the skin barrier, they interact with skin proteins and lipids, inducing skin irritation. On the other hand, when the concentration of surfactant monomers reaches the CMC, micelles are formed, which have relatively larger sizes and lower surface activity (50). Consequently, they lack the ability to penetrate into the skin barrier. The monomer penetration model had been investigated by many researchers, including Ghosh and Blankschtein (51). Sodium cocoyl isethionate (SCI) was studied because it was considered as a mild surfactant. Past studies demonstrated that SCI did not induce serious irritation compared with other anionic surfactants (52). Ghosh and Blankschtein (51) recorded the shift of skin electrical current versus SCI concentration, which did not change further beyond the CMC of SCI. This clearly indicated that the SCI micelles lack the ability to penetrate into the skin. THE SURFACTANT MICELLE AND THE SUBMICELLE PENETRATION MODEL The surfactant monomer penetration model suggests that the penetration of the surfac- tant into the skin is dose independent—the amount of surfactants presented in the skin would not increase further when the surfactant concentration exceeds the CMC. However, skin penetration of SDS showed contradicting results. Moore et al. (50) studied the rela- tionship between the SDS concentration and the amount of SDS penetrating into the skin. The results demonstrated that when the concentration of SDS was beyond the CMC, the amount of SDS in the skin barrier still increased without any limitation. As a result, the penetration behavior of SDS could not be simply explained by the monomer penetra- tion model (53). Through the experiments directed by Ghosh and Blankschtein (51), they found out that the SDS micelles had the ability to penetrate into the skin. Moreover, SDS skin penetration beyond the CMC was predominated by the SDS micelles. This is proposed as the surfactant micelle penetration model.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)