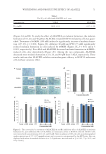
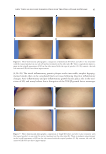
NEW TOPICAL SILICONE FORMULATION FOR TREATING STRIAE DISTENSAE 87 Aloe brings about its effect by promoting infl ammatory cell infi ltration, angiogenesis, extracellular matrix deposition, and epithelialization (35). La st, Centella asiatica is very effective in wound healing and also has been shown to improve SD (18,19). It is used in traditional medicine as a treatment for wounds and scars (38). When applied to wounds, the extract increases cellular hyperplasia, collagen production, epithelization, and angiogenesis, which accelerate collagen cross-linking, reepithelialization, wound maturation, and wound contraction, thus shortening the wound-healing process (39). On a molecular level, asiaticoside, an active ingredient of Centella asiatica extract, suppresses fi broblast proliferation, type I and type III collagen and mRNA expression, and TGF-βRI and TGF-βRII expression and increases Smad7 protein expression, which collectively suppress excessive scarring. Altho ugh our formulation shows an improvement in SD, it does not completely eradicate them. Invasive procedures such as lasers, microneedling, and radiofrequency devices can potentially improve the appearance of SD more so than topical creams. However, there are downsides to such procedures such as the need for repeated procedures, high costs, discom- fort or even pain during the procedure, and postprocedure complications such as swelling, redness, and PIH. Dover et al. (40) reported over 87% improvement in the appearance of SD in those who underwent six repeated sessions of radiofrequency treatment. However, postprocedure erythema and edema remained an issue (6,8). Other studies report up to 90% improvement in SD with either nonablative or ablative CO2 fractional laser during three treatment sessions. However, nearly 82% reported PIH after procedure in the abla- tive CO2 fractional group and experienced, on average, moderate pain during the proce- dure (11). Such high incidence of unwanted procedural sequela further supports the use of topical creams as a fi rst-line agent to improve SD. There are limitations to this study. The fi rst limitation is that the participants were not blinded to treatment, which could lead to a bias toward the treated side. However, our two independent evaluators were both blinded, which increased the validity and reliabil- ity of the study, and saw an improvement in the side treated with the topical formulation similar to what the patients reported. In ad dition, we did not evaluate whether patients would have a reduction in formation of new SD while using the cream. We only evaluated the improvement of their current stretch marks over time. We are aware of prior studies that showed a reduction of SD in pregnant women. Mallol et al. (18) demonstrated a reduction in SD from 56% in preg- nant women who did not use the product to 34% in those who used the topical formula- tion that included Centella asiatica. A reduction in the intensity of SD that were seen in those who were using Centella asiatica was also noted in this study. Another study com- pared topical aloe vera to a placebo-based cream in pregnant women and found that the side on which aloe vera was used had only an 8% progression of SD in comparison to 65% progression of SD during pregnancy with base cream (16). This is an area of interest for future studies using our product. Altho ugh that we did not evaluate our product in pregnant women, our topical product has been shown to be safe in our study. Furthermore, silicone cream and our other ingredients have all been shown to be safe in patients in prior studies with no adverse sequelae. This product should have no contraindications for pregnant women and young adults with SD. We on ly had 1-mo follow-up period, but our study showed that there was a signifi cant improvement in just 1 mo of using the topical product. Furthermore, nearly 90.9% of
JOURNAL OF COSMETIC SCIENCE 88 our patients wished to continue using the product after the 1-mo period. Future research should follow the cohort for longer than 1 mo to uncover whether longer use of this prod- uct could result in potentially better outcomes. Last, some may argue that the formulation’s moisturizing vehicle by itself could induce enhanced moisturization of the skin and improve the appearance of SD. Future studies should use a split study of our formulation versus a placebo moisturizer. CONCL USION This study demonstrates that the investigated topical formulation is safe and effective to use for SD. It can help improve the appearance and texture of SD. It may also be consid- ered for use in helping to prevent SD, as prior evidence supports the use of topical creams in preventing the formation of new SD, especially in pregnant women. Autho r Disclosure: Christopher I. Zoumalan, MD, owns stock and is the scientifi c advisor for MD Medical Designs, Inc., Los Angeles, CA, manufacturer of the stretch mark cream used in this study. ACKNO WLEDGMENT We th ank Cristina Luna for her research-coordinating support. REFER ENCES (1) A . L. S. Chang, Y. Z. Agredano, and A. B. Kimball, Risk factors associated with striae gravidarum, J. Am. Acad. Dermatol., 51(6), 881–885 (2004). (2) K. H. Kelekci, S. Kelekci, E. Destegul, A. Aksoy, N. Sut, and B. Yilmaz, Prematurity: is it a risk factor for striae distensae? Int. J. Dermatol., 50(10), 1240–1245 (2011). (3) A. Hague and A. Bayat, Therapeutic targets in the management of striae distensae: a systematic review, J. Am. Acad. Dermatol., 77(3), 559–568 (2017). (4) B. J. Kim, D. H. Lee, M. N. Kim, K. Y. Song, W. I. Cho, C. K. Lee, and O. S. Kwon, Fractional pho- tothermolysis for the treatment of striae distensae in Asian skin, Am. J. Clin. Dermatol., 9(1), 33–37 (2008). (5) S. Kang, Topical tretinoin therapy for management of early striae, J. Am. Acad. Dermatol., 39(2), S90– S92 (1998). (6) M. C. A. Issa, L. E. de Britto Pereira Kassuga, N. S. Chevrand, L. do Nascimento Barbosa, R. R. Luiz, L. Pantaleão, E. G. Vilar, and M. C. Rochael, Transepidermal retinoic acid delivery using ablative frac- tional radiofrequency associated with acoustic pressure ultrasound for stretch marks treatment, Laser Surg. Med., 45(2), 81–88 (2013). (7) S. Guida, M. G. Galimberti, M. Bencini, G. Pellacani, and P. L. Bencini, Treatment of striae distensae with non-ablative fractional laser: clinical and in vivo microscopic documentation of treatment effi cacy, Laser Med. Sci., 33(1), 75–78 (2018). (8) V. Mishra, L. Miller, S. M. Alsaad, and E. V. Ross, The use of a fractional ablative micro-plasma radio- frequency device in treatment of striae, J. Drugs Dermatol. JDD, 14(11), 1205–1208 ( 2015). (9) H. Bak, B. J. Kim, W. J. Lee, J. S. Bang, S. Y. Lee, J. H. Choi, and S. E. Chang, Treatment of striae distensae with fractional photothermolysis, Dermatol. Surg., 35(8), 1215–1220 (2009). (10) F. Malekzad, S. Shakoei, A. Ayatollahi, and S. Hejazi, The safety and effi cacy of the 1540nm non-ablative fractional XD probe of star lux 500 device in the treatment of striae alba: before-after study, J. lasers Med. Sci., 5(4), 194 (2014).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)