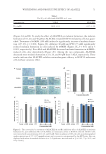
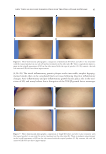
J. Cosmet. Sci., 71, 77–90 (March/April 2020) 77 Safety/Effi cacy of New Topical Silicone Formulation with Selective Growth Factors for Treating Striae Distensae ROBIN KIKUCHI, ANDREA R. WALDMAN, ERIK M. WOLFSWINKEL, VADIM PLETZER, BRANDON D. KALASHO, and CHRISTOPHER I. ZOUMALAN , Aesthetic and Reconstructive Oculoplastic Surgery, Beverly Hills, California 90212 (R.K., A.W., B.K., C.I.Z.), Department of Plastic Surgery, Keck School of Medicine, University of Southern California, Los Angeles, California 90033 (E.M.W.), Division of Applied Statistics, Victoria University of Wellington, Wellington, New Zealand 6140 (V.P.) Accepted for publication February 10, 2020. Synopsis Striae distensae (SD) are linear dermal scars that arise from progressive stretching or tearing of the dermal layer. This study tests the safety and effi cacy of a topical formulation of silicone-based scar cream containing selective synthetic recombinant human growth factors, hyaluronic acid, and vitamin C to improve overall appearance and texture of SD. Twenty-two subjects with SD alba were recruited and randomized to apply the topical formula to half of their SD laterally twice a day for 1 month. Patient surveys were obtained at 1 month for overall appearance, texture, and tolerability. Three-dimensional imaging was obtained at baseline and at 1 month and submitted to independent evaluators for grading on overall appearance. Subjects reported improved texture and appearance in 86.4% of SD. Subjects reported 100% of untreated SD to have no change in overall appearance or texture. 90.9% of subjects reported no tolerability issues. 9.1% of the treated group reported mild issues such as slight itching or redness the fi rst day of application, which subsided in 2 days for all patients. Independent evaluators indicated improvement in 72.7% of SD in comparison to improvement in 36.3% of untreated SD. This study demonstrates that the investigated topical formulation is safe and effective to use for SD. INTRODUCTION Striae distensae (SD), or stretch marks, are linear dermal scars that arise from progressive stretching or tearing of the dermal layer. They are a cosmetic concern to a signifi cant part of the general population, occurring in 50–90% of the general population following rapid weight changes during adolescence, pregnancy, or with corticosteroid use (1–3). The commonly affected areas include the abdomen, outer thighs, buttocks, axilla, and breasts. SD progress through three stages. They fi rst appear as red infl ammatory, pruritic, Address all correspondence to drchris@zoumalanmd.com .
JOURNAL OF COSMETIC SCIENCE 78 and slightly raised striae, termed SD rubra. These then progress into a purple hue, termed SD purpuric. Finally, they progress to a chronic form where they appear hypopigmented and atrophic. This end state is often termed the fi nal scar and referred to as SD alba (3–5) . Although they do not cause signifi cant medical problems, aesthetically, SD can be a cause of great concern or psychological stress for many individuals. Thus, a variety of modalities have been attempted to treat SD, but there is no exemplar treatment that is currently available. Currently available treatment options range from topical creams to more invasive procedures, such laser therapy, microneedling, and radiofrequency treatments. These in- vasive procedures have been shown to improve the appearance of SD to a variable degree, but they are often costly and require multiple treatment sessions at a physician’s offi ce, which can result in postprocedure downtime for the patient. Furthermore, complications associated with these treatments can include postinfl ammatory hyperpigmentation (PIH), which is common with lasers, as well as redness, pain, and edema, which are com- mon issues with lasers, microneedling, and other devices such as radiofrequency (6–12) . From a practical standpoint, considering the cost of and access to treatments, topical creams are often the fi rst-line treatment for SD in many patients (3). As a result, there is a multitude of topical products on the market, and many claims to improve the appear- ance of SD without any scientifi c evidence. Of particular note, there is some evidence in the literature to support the use of specifi c topical ingredients for reducing SD. For instance, ingredients such as selective growth factors, aloe vera, Centella asiatica, hyaluronic acid (HA), vitamin C, and silicone cream have all been shown to improve SD and postsurgical scars (12–22). In fact, a recent topical formulation using the ingredients mentioned ear- lier was used to evaluate postsurgical scars in a head-to-head double-blind prospective randomized multicenter trial. The fi ndings demonstrated this formulation was 73% more effective than generic silicone cream in improving the appearance of scars (23) . Given the knowledge that SD are similar histologically to scars and often termed a der- mal scar, we hypothesize that this same formulation that was shown to improve scars nearly 2 times better than silicone cream in postsurgical scars may provide improvement in the appearance of SD. In this study, we evaluate the safety and effi cacy of a proprietary product that includes selective growth factors and other ingredients such as aloe vera, Centella asiatica, HA, and vitamin C within a silicone cream in treating SD. METHODS This study investigates the safety and effi cacy of a proprietary product in treating SD. The topical formulation consists of synthetic recombinant TGF-β3, HA, aloe vera extract, Cen- tella asiatica extract, oil-soluble vitamin C, and several other synthetic recombinant hu- man growth factors that are implicated in the wound-healing process. The silicone portion consists of dimethicone 10%. The topical product is manufactured by MD Medical Designs, Inc. (Beverly Hills, CA). The protocol for the study was conducted according to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Subjects older than 18 years with SD of the abdomen, thighs, or buttocks were included in this study. Subjects with bothersome SD who were existing patients in the medical offi ce of author C. I. Z. were included. Patients who had received previous treatments for the same SD, including laser resurfacing, radiofrequency, microneedling, or other topical treatments were excluded. In addition, female patients who were pregnant or breast feeding
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)