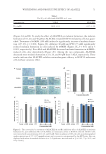
NEW TOPICAL SILICONE FORMULATION FOR TREATING STRIAE DISTENSAE 83 Table VI Evaluator Average Scores for Overall Appearance Treatment Score Proportion (%) N = 22 patients 95% margin of error Topical product Worsened 0/22 (0.0%) ±0.0% No change 6/22 (27.3%) ±13.2% Mild improvement 6.5/22 (29.5%) ±13.5% Moderate improvement 7.5/22 (34.1%) ±14.0% Signifi cant improvement 2/22 (9.1%) ±8.5% No treatment Worsened 0.5/22 (2.3%) ±4.4% No change 13.5/22 (61.4%) ±14.4% Mild improvement 6.5/22 (29.5%) ±13.5% Moderate improvement 1.5/22 (6.8%) ±7.4% Signifi cant improvement 0/22 (0.0%) ±0.0% Figure 4. Evaluator overall a p pearance: Average distribution of scores. were rated as having better overall appearance. This was verifi ed to be a statistically sig- nifi cant difference by the Wilcoxon signed-rank test (Table VII, Figure 5). No adverse reactions were recorded over the duration of this study, and no patient discon- tinued the use of the topical product. DI SCUSSION Ba sed on evaluator grading, we saw improvement in 72.7% of treated SD over a period of 1 mo with our topical product. In addition, the majority of subjects (86.4%) indicated that they saw mild improvement or more to their treated SD (Figures 6–8). No subjects indicated a worsening in appearance of SD or intolerability of the product. Based on this study, the formulation was safe to use and well tolerated.
JOURNAL OF COSMETIC SCIENCE 84 Al l of these ingredients used in the product we evaluated have been individually shown to improve SD in prior literature and, now, when collectively used in our topical formula- tion, SD improved signifi cantly within 1 mo of use. We are unable to discern if there is a specifi c ingredient that may play a dominant role in the formulation, but it is most likely the improvement we saw is because of the synergistic effect of all the ingredients working along the surface of the skin. Th ere is evidence to support the use of silicone gel in treating SD. A recent study found that SD treated with silicone over a placebo cream had a statistically increased collagen and reduction in pigmentation (13). In addition, 2 recent studies demonstrated that silicone-based products improves SD in pregnant women (9,21). SD have histological similarities to scars, and silicone, which is used as the foundational matrix of our product, has a long track record as a scar-reducing treatment (24). In clinical trials, silicone has demonstrated effectiveness in preventing hypertrophic or keloid scarring in patients with newly healed wounds (25). I n addition, growth factors play a role in the progression of SD. A recent study indicated the positive effects of using growth factors to manage SD (12). Moreover, from a histo- logic standpoint, SD formation is similar to wound-healing processes found in scars Table VII Evaluator Average Scores for Overall Appearance Side Proportion (%) 95% margin of error Topical product better 19/22 (86.4%) ±14.3% Same 3/22 (13.6%) ±14.3% No treatment better 0/22 (0.0%) ±0.0% Figure 5. Evaluator average sc o res for overall appearance of topically treated SD.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)