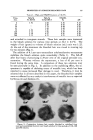
142 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS • ?"' . .*." "•t, •%.*.. %.. A • M•..• . •,, •.. • . :• " . :•%•' '" •2 .• :•' ' "•".•.'•-• . •.•....-• .... • •F' • :f .::..•' ..... :½.'• f. • • •.. ...... Z•' ... ... .:•:'.•'"• '" ., '•?•: X ... .•"'., :• ' .. •...s."'•.% ......... ' "'*•4.•c. •. '•::' '•: :,: .... ? '..• ' ' . ...7." .. :. '**'•Z• •.'" .•'. .,•.. .:,• .... ......... ...,..?.-•,• .. •: •*.•i•.. • &.•* , :• -. ,q• . . ::. -.. .:.:½,. .,,.•....._. Figure 7.--Shows the degree ofoxid•tio• d•*•ge o• the h•ir, •s c• be seen by the green color. 50 mi. distilled water. The copper in the tiltrate and washings was de- termined as mentioned above. The copper uptake of the fibers is calcu- lated as a percentage of the origi,,1 fiber weight. It has been reported that on wool oxidation damage results in a green 4.0 HaOa + Cu-SORPT!I•N ON BLEACHED HAIR H•,Oz •.c •q. I leo •sb TIME IN MINUTES Figure 8.--rl•he bottom curve shows the copper uptake in per cent, plotted against bleaching time. The upper curve shows hydrogen peroxide sorption plotted against bleaching time.
PROPERTIES OF PEROXIDE-BLEACHED HAIR 143 color, alkali damage in a gray color and acid damage in a violet color. In these experiments on the human hair, the color was green, indicating oxidation damage very clearly (Fig. 7). If the damage scale (Table 6) for wool is used, it would have to be said that damage starts at approx- imately thirty minutes. In this work, the green copper color was visible even on the untreated hair. From the curve in Fig. 8 we can see that the copper-uptake test is only useful for indicating small damages to the fiber. Hydrogen Peroxide Sorption To further understand the physico-chemical properties of hydrogen peroxide bleached hair, peroxide sorption studies were conducted. The sorption studies of hydrogen peroxide on hair are based on the centrifugal method developed and described by Valko and Barnett in their swelling studies (12). Five hundred mg. hair samples, kept at 21øC., and 65 per cent relative humidity, were placed in stainless steel crucibles. Twenty-five ml. phos- phate buffer (pH 4) was added and the fibers thoroughly wetted by stirring for five minutes. The buffer solution was removed by suction, and the samples centrifuged for ten min- TABLE 6.--CoPPEP` UPTAKE AND DAMAGE SCALE FOP. BLEACHED WOOL Time, min. 0 15 30 45 Copper, % 1.76 2.17 2.49 2.54 Time, min. 60 120 180 240 Copper, % 2.90 3.05 3.34 3.42 DAMAGE SCALE FOP. WOOL 0.0-1.0 Not damaged 1.0-1.5 Very slightly damaged 1.5-2.5 Slightly damaged 2.5-3.8 Damaged TAm, E 7.--HYDP. OGEN PEP.OXIDE SOP.PTION Time, H•O2, min. % 0 3.30 60 3.49 120 3.55 180 3.64 240 3.71 utes at 2210 r.p.m. corresponding to a relative force of 1000 gravities. After that the crucibles were filled with 6 per cent H202 solution (ad- justed to pH 4), the fibers thor- oughly wetted by stirring for five minutes, after which time the excess was taken off by suction and the crucibles submitted for ten minutes to 1000 gravities. The hair samples were then transferred into 250 ml. titration flasks. One hundred ml. distilled water and 10 ml. H2SO4 (1:4), and 0.1 ml. Ferroin were added. The H202 content was de- termined with 0.1 N ceric sulfate solution. The sorption of H202 by wool has been reported and is fully described by Alexander and co-workers (13). It is concluded from their work that H=O2 is physically held by amino and carbimino groups. The above experiments show a slight increase of HsOs sorption with increased bleaching time (Table 7 and Fig. 8). It seems, therefore, that the sorption of HsO2 reflects to some extent the de-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)