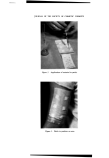
260 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS NH N•-t CI .NH.C, NH.C. NH.(CH•)•oNH, C. NH. Co NH. NH H • CI. Figure 1. Chemical structure of ehlorhexidine available commercially as gluconate solution (B.P.), acetate (B.P.C.) and hydrochloride (B.P.). must be few hospitals in the British Isles and Commonwealth in which it is not used in one form or another. A multiplicity of simple and complex compositions have been investigated by various professions in turn-- formulators, practising pharmacists, bacteriologists, dermatologists, sur- geons, urologists, gynaecologists and ophthalmologists, and the overall conclusion is that chlorhexidine is at the forefront of modern antibacterial agents because of its broad spectrum, rapid action, effectiveness at high dilution, innocuous nature and low incidence of resistant strains. SELECTION OF SALTS When a drug exerts basic properties it is often a rewarding policy to examine a comprehensive series of salts of various chemical types, studying the requirements of all interested parties before reaching a decision on which ones to manufacture. In the first place, the development chemist seeks the salt which is most readily isolated and his choice is therefore governed by the ease with which it is crystallized to give high purity and good yield. The formulator views the subject from a different aspect in seeking a salt of low solubility in water for some applications, for instance those requiring prolonged antibacterial action with only a limited concentration of avail- able antibacterial in contact with the tissues (depot effect). Too low a solubility, however, may lead to difficulty in isolating a pure salt and also to inadequate release of active agent. On the other hand, and particularly when swift bactericidal effect is sought, an extensively soluble salt is a prime objective. The practising pharmacist making his own formulations prefers a rapidly and extensively soluble salt for convenience and economy in pro- viding strong aqueous concentrates, while the manufacturer of proprietary compositions has a similar attitude, seeking rapidly to prepare fluid formula- tions in bulk at ambient temperatures. Having regard to these several re- quirements many chlorhexidine salts were eaxmined, of which Table I shows a selection. There were two main methods of preparation•doublo
FORMULATION AND PROPERTIES OF CHLORHEXIDINE 261 decomposition of chlorhexidine acetate in warm aqueous solution with the appropriate sodium salts to make the sparingly soluble salts, and dissolu- tion of the base in the appropriate acids, in aqueous solution, for the more soluble salts. An alcoholic solution of the acid was occasionally used, ac- cording to circumstances. The base was a white crystalline solid m.p. 132øC which, like its salts, was colourless, odourless (see exception) and with a bitter taste. Aqueous solutions saturated at 20øC were analysed by the colorimetric method of Holbrook, a reddish-brown colour developing by reaction with alkaline hypobromite (3). Chlorhexidine functioned as a di-acid base in all these circumstances, though tetrasalts could be prepared with certain strong acids which, not unexpectedly, gave rise to acid solutions. For sim- plicity the prefix 'di-' has been dropped from the official B.P. and B.P.C. titles. The inorganic salts proved to be of remarkably low solubility, fore- boding trouble with hard water, and in particular with the sulphate ion. The dihydrochloride salt was chosen from this series as the most con- venient sparingly soluble salt for medical usage. The lower aliphatic acids proved to be more soluble and led to the selection of the diacetate, not an ideal choice, as it was difficultly and inadequately soluble for certain concen- trates. It also gave an odour of acetic acid in aqueous cream formulations. At a later date the subject was re-examined and, noting that hydroxylation improved solubility (for instance, dipropionate 0.4•o, dilactate 1.0•o), several polyhydroxy acids were tested, particularly the sugar adds, and this Table I Chlorhexidine salts--water solubilities at 20 ø C Salt % w/v Salt % w/v Salt % w/v (Base) 0.008 Diformate 1.0 Dilactate 1.0 Dihydriodide 0.1 Diacetate 1.8 Di-o•-hydroxyiosbutyrate 1.3 Dihydrochloride 0.06 Dipropionate 0.4 Digluconate 70 Dihydrofluoride 0.5 Di-i. wbutyrate 1.3 Diglucoheptonate 70 Diperchlorate 0.1 Di-n-valerate 0.7 Dimethanesulphonate 1.2 Dinitrate 0.03 Dicaproate 0.09 Di-isethionate 50 Dinitrite 0.08 Malonate 0.02 Dibenzoate 0.03 Sulphate 0.01 Succinate 0.02 Dicinnamate 0.02* Sulphite 0.02 Malate 0.04 Dimandelate 0.06 Thiosulphate 0.01 Tartrate 0.1 Di-isophthalate 0.008* Di-acid phosphate 0.03 Dimonoglycolate 0.08 Di-2-hydroxynaphthoate 0.014' Difluorophosphate 0.04* Monodiglycolate 2.5 Embonate 0.0009* *These are approximate values.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)