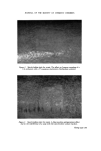
264 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS B.P.C., in a 0.1•o chlorhexidine gluconate solution was studied by Barnes (9) using the inhibitory zone method, in duplicate, to assess the available activity. It was clear that the adverse effect of the surfactant increased greatly with concentration though in practice the chlorhexidine strengths are usually higher than that of the surfactant. The loss in availability at equal concentra- tions (0.1 •o each in Fig. 2) was about 30•o, not too serious a matter with so potent an antibacterial agent. However, a paraffin-water emulsion contain- ing 0.1 •o chlorhexidine gluconate with cetostearyl alcohol and LubroI W as the co-emulsifiers lost over 90•o of its apparent availability when the latter was at 1 •o strength, thus illustrating the importance, as with liquid prepara- tions, of keeping surfactants to the minimum concentration consistent with long-term stability. A modification of the above experiment on creams in which the LubroI W was held at 1•o, varying the chlorhexidine gluconate between 0.1•o and 1.0•o, is summarized in Table II. From Table 11 it seems that an o/w cream containing 1 •o chlorhexidine gluconate and 1 •o Lubrol W shows only about 20•o activity. It was found in a control test with an aqueous solution of the same strength that there was about 40•o available chlorhexidine, and reference to Fig. 2 reveals that a tenfold aqueous dilution (i.e. 0.1•o each) gives nearly 70•o availability. Thus, it appears that the liquid preparations are more effective than corre- sponding creams. As chlorhexidine gluconate is insoluble in fats it is there- fore mostly present in the aqueous phase and similarly Lubrol W is known 7O BO 5O 40 :50 2O 0 I I I I I I I I I I I I .... l o i 2 3 4 % oeubrol W Figure 2. Effect of a polyoxyethylene glycol monoalkyl ether on availability of chlorhexidine gluconate. O-- O, 0.1% w/v chlorhexidine gluconate in water O-- O, 0.1% w/v chlorhexidine gluconate in o/w cream.
FORMULATION AND PROPERTIES OF CHLORHEXIDINE 265 Table II Effect of Lubrol W (1% w/w) on chlorhexidine availability in an o/w cream Available chlorhexidine Chlorhexidine Expressed as % gluconate concentration of total Expressed as % (% w/w) concentration Test 1 Test 2 Average 0.10 8.1 6.4 7.2 0.0072 0.37 14.3 14'9 14.6 0.0054 0.73 19.2 19.6 19.4 0.1500 1.00 18.5 20.5 19.5 0.2000 to preponderate in this phase. One possible explanation for these differences is that the chlorhexidine may be held by the surfactant micelie. Another alternative is that the higher viscosity of the cream accounts for the reduced diffusion through the agar medium. One surfactant commonly used with aqueous chlorhexidine antiseptic solutions is Lissapol NXU. (Note: this should not be confused with Lissapol NX, which may cause precipitation.) Fig. 3 demonstrates its effect over a 0.8 0,7 0.6 0.5 0.4 / If no interactian 0.3 / 0.2 / / / o.I / o o.I 0.2 0.3 o,4 0.5 0.6 o.7 % w/v 'free' chlorhexidine acetate Figure 3. Effect of surfactants on chlorhexidine availability. Open symbols, Lissapol NXU 0, polysorbate 80.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)