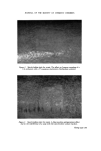
266 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS range of concentrations. Hall (10) determined the chlorhexidine acetate in rhe 'free' form by the in vitro bactericidal effect on Staphylococcus aureus. Polysorbate 80 was likewise tested and apparently gave rather better results at 15/o though this might be of. little significance in practice. In a further experiment to determine the influence of' polysorbate 80 in excessive proportion the presence of 1.0•o and 3.35/0 of this surfactant was shown to reduce the activity of a 0.1•o chlorhexidine acetate solution to 39•o and 14• respectively. Corresponding figures for Lubrol W given above for the gluconate were 9•o and 55/0. Without reading too much sig- nificance into these differences the fact emerges that a series of candidate surfactants should be compared over a range of concentrations for their influence on antibacterial availability and specific bactericidal action against the appropriate organisms. Hugo and Longworth (11) have suggested that the mode o[' action of. chlorhexidine is to danaage the permeability barrier of the bacterial cell, while Wiseman (12) considered that the two effects of chlorhexidine, viz. the disruption of the permeability barrier of the cells and the blocking of. elec- tron transport in the cytochrome system were both attributable to chlor- hexidine combining with the cytoplasmic membrane and causing an altera- tion or breakdown of its structure. In such circumstances it would be expected that a surfactant might further affect the cell wall, thereby enhancing the effect of the chlorhexidine. Brown and Richards (13) were able to demon- strate this action in cultures of Pseudomonas aeruginosa containing poly- sorbate 80, the organism being much more susceptible to chlorhexidine (also to benzalkonium chloride and polymyxin B sulphate) than cells grown in plain broth. Aqueous solutions of chlorhexidine, unless sterilized by a heating pro- cess or by filtration, may occasionally become contaminated with water borne Pseudomonas species. The lower aliphatic alcohols n-propanol (4•o v/v), isopropanol (45/o v/v) and ethanol (75/0 v/v) are valuable adjuncts in this context and such preparations are found to be free from vegetative bacteria even though prepared with heavily contaminated water. The possible influence of ethanol on a simple aqueous solution containing chlor- hexidine and polysorbate 80 was investigated by Heard (14)using Visking dialysis tubing through which chlorhexidine diffused very much more quickly than polysorbate 80. Results are given in Fig. 4. The effect of ethanol was to reduce the interaction of chlorhexidine with the surfactant to an extent related to its concentration. Even at the low strength of 10•o ethanol it appeared that the activity of chlorhexidine acetate in a 0.04•o solution with
FORMULATION AND PROPERTIES OF CHLORHEXIDINE 267 0,05 O,04 0.03 0,02 I I I 0.01 0,02 0.03 0,04 'free' chlo•hex•dine c•½etote Figure 4. Effect of ethanol (v/v) on chlorhexidine acetate in 1% w/v aqueous polysorbate 80. O, 0% ethanol O, 10% ethanol &, 20% ethanol E, 50•o ethanol. 1 •o polysorbate 80 was increased by almost 50•. A previous worker, Becher (15), had shown that ethanol raised the critical micelle concentration of a polyethylene lauryl alcohol, preventing micelle formation at 25• strength. In the experiment with chlorhexidine, 50• ethanol evidently exerted a high suppressive action on the suffactant, probably for a similar reason. SYSTEMIC TOXICITY The acute oral toxicity of chlorhexidine is low, presumably due to its low systemic absorption, for it is almost entirely excreted in the faeces. In small animals the LD 50 is 2 g kg -• (acetate) or 1.8 g kg -• (gluconate). Chronic toxicity tests on rats fed with 0.05• chlorhexidine acetate showed no ill- effect and weight gain was normal. A monkey tolerated the same procedure for 6 months, also with no ill-effect. Humans have taken 2 g daily for a week without any untoward symptoms. The long-term prophylactic usage of chlorhexidine in dental conditions, a very recent development, has called for a more protracted study of chronic toxicity in its many aspects and re- search in this field is now receiving very close attention.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)