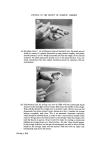
196 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The same column is used as for the aromatic diamines. (See hair colourants.) Benzocaine gives a good response. Not all of the reference sunscreens gave a response, but that was unimportant, as glc matching was the main purpose. Chemical analysis of sunscreens Group reactions for p.aminobenzoic acid and esters, and salicylate esters were carried out. P.aminobenzoic acid/esters. Ehrlich reagent (1 •o dimethylaminobenzaldehyde in 10•o HC1) gives a strong Yellow/Orange colour. Application directly on the sample or as a spray on a tlc plate. N.B. Many other compounds which are non-uv absorbers with aromatic amine groups show a positive Ehrlich reaction, e.g. sulphonamides. The possibilities for sunscreens are: p.Aminobenzoic acid, ethyl p. Amino- benzoate (= benzocaine), glyceryl p.aminobenzoate. N.N.dimethyl p.amino- benzoic acid esters do not give a reaction. Salicylic acid esters. After saponification, the free salicylic acid will give a violet colour with 1 •o FeCla. Experimental. Boil 0.5 g sample with 5 ml 8•o ethanolic KOH under reflux from 30 min. Cool, dilute with 40 ml water. Neutralize to pH 5-7. Add 1 drop 15/o FeCla. A violet colour appears, which will persist after the addition of an equal volume of ethanol (phenol gives a violet colour that fades with ethanol). General problems By means of a simple tlc/glc matching many of the sunscreens could be identified. An example is given in Fig. 10. Not all the sunscreens could be identi- fied, mainly because the range of reference compounds was not sufficient. Benzocaine in sunscreens In several samples of two brands, the analytical data indicate the presence of benzocaine. This substance has sunscreen properties, but acts as a local anaes- thetic as well. The presence of benzocaine was confirmed by its ir spectrum and its glc response, after isolation by column chromatographic means. Identification of benzocaine (ethyl p.aminobenzoate). Two samples (1 and 48) gave a strong yellow/orange coloration with Ehrlich reagent. Such a positive reaction, however, might also be given, not only by benzocaine, but also by the free p.aminobenzoic acid or its glyceryl ester, both of which are known sunscreens. Tlc separation of these three substances was achieved with the following solvent system:
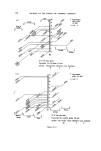
RISK-BEARING SUBSTANCES IN COSMETICS 197 tic Sam •Ie no. o oo o oo • o o ß 00¸ 0 S tort el oO ß ß g lc: relahve retenhon hmes (Benzo(•mne:) 1,5 1 UI, 9 14,7 0,7 2 • 2 ,G I 5,30 "51 D 1'9 14,7 0,72 • 2,6 0,600 i 00,90 D 5,21:] 10,72 i]1,3 rl 2,6 i i i i 511• Start 1,0 2,0 5,0 4,0 5,0 IO, 72 1,5 II, I 4,8 I I,,5 12,7 I Figure 10. Example of tlc/glc matching for the identification of sunscreens. Sample no. 10 contains B (Givtan F). Sample no. 11 contains A (Eusolex 3573) and possibly C (Solprotex 1). Sample no. 12 contains B (Givtan F). Sample no. 13 and 14 contain A (Eusolex 3573) and possibly C (Solprotex 1). Diisopropylether-n. Hexane-Acetic acid 75 ß 35 ß 1, by volume. Visualization was with Ehrlich reagent. Sample no. Rf 1 48 Benzocaine 0.55 + + p.Aminobenzoic acid 0.45 -- + Glycerol ester of pAB 0 -- + Saponification of both samples, followed by extraction and tic, left only one spot of the free acid. Procedure: Boil « g of the sample with 5 m183/0 ethanolic KOH during 30 min. Cool and dilute with 20 ml water. Acidify with 4M HC1 to a pH of 1-3. Extract the free acid with 2 x 10 ml chloroform. Evaporate the chloroform fraction to 1 ml, and use this for tlc. Benzocaine was isolated from both samples by means of column chromato- graphy. The isolated fractions of Benzocaine (Fraction I, 4 of sample no. 1 and Fraction I, 3 of sample no. 48) were confirmed by its ir spectra and its response on glc. Procedure: Use 2 x 20 cm glass columns. Fill with 15 g Silicagel Merck (diam. 0.05-0.20 mm) suspended in n. Hexane. Add a mixture of (1 g sample + 1 g Na.,.SO• exsicc. + 5 ml n. Hexane) on the column. Elution proceed as described below. The column fractions are concentrated and controlled by tlc (as above).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)