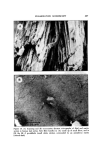
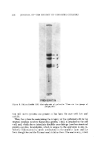
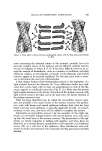
CELLULAR CEMENTING SUBSTANCES 435 tein has been shown to be a helical molecule, similar, but not identical to the classical a helix (Fig. 2). The a proteins have been most extensively studied in cow snout epidermis (1, 2, 3). The a protein of the viable epidermis, prekeratin, can be solubilized by urea or buffers of organic acids below pH 2.7. Purification from acid buf- fers can be achieved by isoelectric precipitation. This material is insoluble in pH range 3-10, but can be maintained in solution at neutral pH by the addi- tion of urea, guanidine, or sodium dodecyl sulfate (SDS). The analysis of amino acid reveals a high content of the acidic amino acids and glycine (Table I). The cystinc content is quite low, unlike the a protein of hair and nail. In SDS electrophoresis has shown that a number of components (Fig. 3), and immunologic studies indicate that the A and B families are distinct from one another, but both are necessary to form an a helix. These results are best interpreted as the prekeratin molecule consisting of 3 polypeptides, 2 A chains and 1 B chain. It would appear that a major prekeratin exists with the A, A', B chains and a minor one exists with an A, A', B' chain. No cystinc cross-links occur between the polypeptides of prekeratin. As the cells of the viable epidermis become cornified at the base of the stratum corncure, the a fibrous proteins become cross-linked and can only be solubilized by alkaline buffers that contain a denaturing agent such as urea and a reducing agent (4, 5). This process is irreversible, and the stratum cor- neum fibrous proteins become cross-linked when the reducing agent is re- moved. The amino acid composition of the stratum corneum reveals a one- half cystinc content of 2 residues/100 residues indicating that no one-half cys- Table I Amino Acids Prekeratin Lysine 15.1 Histidine 1.0 Arginine 6.1 Aspartie acid 9.1 Threonine 4.0 Serine 11.1 Glutamic acid 14.1 Proline 1.4 Glyeine 16.4 Alaninc 6.7 Valine 4.0 Methionine 1.3 Isoleueine 3.5 Leucine 9.2 Tyrosine 2.8 Phenylalanine 3.6 Half cystinc 0.6
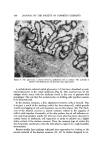
436 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS P :REK E RAT I N Figure 3. Polyacrylamide SDS electrophoresis of prekeratin. There are two groups of polypeptides tine rich matrix proteins are present as has been the case with hair and nail (6). Thus, the system for maintaining the integrity of the epidermal cells in the stratum corneum involves filamentous protein, which is attached to the cell wall, and, which shows interchain disulfide cross-linkage (another structural protein complex, keratohyalin, which is unique to the epidermis is also in- volved.) This material is newly synthesized in the granular layer and has been thought to coat the filaments and stabilize them. The controversy, which
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)