J. Soc. Cosmet. Chem., 28, 441-446 (August 1977) Antiperspirant efficacy E. S. BRETSCHNEIDER, A.M. RUBINO, and J. J. MARGILES Reheis Chemical Company, Division of Armour Pharmaceutical Company, Berkeley Heights, NJ 07922 Received September 1 O, 1976. Presented Ninth IFSCC Congress, June 1976, Boston, MA. Synopsis ANTIPERSPIRANT EFFICACY of ALUMINUM CHLOROHYDRATE "TYPE" INGREDIENTS is dis- cussed. The optimal efficacy for aluminum chlorohydrate and aluminum bromohydrate in aqueous solution occurs at concentrations of 15 per cent (3.75 per cent A1) and 24 per cent (5.0 per cent A1), respectively. No difference in efficacy between aluminum chlorohydrate-A1Cla combinations and aluminum chl- orohydrate alone, is found. Efficacy differences are observed as a function of vehicle. For example, aqueous formulations appear to be more efficacious than anhydrous formulations. Efficacy of aluminum-zirconium compounds is discussed in terms of variation of Al:Zr ratio. No differences are found. INTRODUCTION Antiperspirants as topical drugs have come under the scrutiny of FDA-OTC panels. Because of this interest and since the definition of antiperspirants is based on their efficacy, a study of the effectiveness of commonly used active ingredients would be useful. Unfortunately, there is a paucity of published information in this area. Recent papers have dealt with experimental designs and statistical interpretation of data (1,2, 3), mostly on formulated products. Since efficacy can be influenced by adjuvants in formulations, we believed an investigation of active ingredients in simple aqueous and nonaqueous vehicles worthwhile. In recognition of this information gap, we have studied the relationship between active ingredient efficacy with both concentration and solvent variations. We hope that this data will enlarge the cosmetic chemist's horizons, in developing new and improved vehicles for the application of a chosen anti- perspirant. To render this study both feasible and meaningful, we limited our investigational ef- forts to aluminum chlorohydrate "types" as well as aluminum-zirconium complexes. Aluminum chlorhydrate, a 5/6 basic aluminum "salt," AI2(OH)sC1, has been used as an antiperspirant for over 30 years (4). Other aluminum salts, such as aluminum chloride, were available as antiperspirants in the early part of the twentieth century, primarily for use by actors and models. The drawback of this product is its high acidity resulting in fabric damage and skin irritation. To circumvent this problem, buffers such as urea were used. Then in the early 1940s an internally buffered product, aluminum chlorohydrate, became available. In recent years, however, aluminum chloride has regained popularity, primarily when used in conjunction with basic aluminum "salts." In a twist of fate, aluminum chlorohydrate, which originally replaced buffered alu- minum chloride systems, is now being used to buffer aluminum chloride, the dif- 441
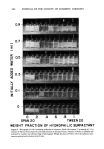
442 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ference being that, in the latter case, 2 active ingredients are used rather than one. Some products which use 2 active ingredients also use a buffer such as urea, amino acid, or an inorganic salt to decrease acidity. Other multi-active systems consisting of aluminum and zirconium salts have also received considerable attention in recent years. The effectiveness of all these products is of critical importance to cosmetic chemists. We will discuss the efficacy of many of these systems and some of their isologs in rela- tion to the effects of concentration and vehicle. For example, one may reasonably ask, "Is the efficacy of aluminum chlorohydrate in aqueous formulations as effective as hydroalcoholic or anhydrous formulations?" We will try to answer questions of this type, but first, a brief review of the clinical procedure will be described. EXPERIMENTAL DATA COLLECTION* The efficacy data were obtained using 0.5 ml applications. A gravimetric method was employed to obtain these data (1). Panelists were required to abstain from the use of all antiperspirant materials from enrollment until completion of test. Sweating of test panelists is induced by having the panelists sit in a room maintained at 100 +_ 2øF and at a relative humidity of 35 per cent. Before collection of perspiration, there is an appropriate warmup period. All data were obtained 22 h after final applica- tion of product. DATA TREATMENT The geometric mean was used to calculate efficacy (2,3). In the statistical analysis, we use logarithmically transformed milligram weights. The per cent reduction is calculated as follows: Per cent Sweat Reduction = [1-antilog (T' - C')]x 100 where T' and C' are the average values of the logarithmically transformed milligram weights for the test (treated) and control (untreated) axillae. RESULTS AND DISCUSSIONS ONE-INGREDIENT FORMULATIONS Dose response curves are normally available for drugs. Little information is available, however, on the variation of efficacy (response) with concentration (dose) for anti- perspirants, when employed as topical drugs. Efficacy data for one of the more popular *Efficacy data obtained from Hill Top Research, Inc., Miamiville, OH. For a detailed account of their method, see (1).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)