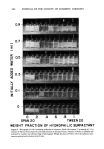
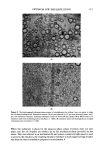
J. Soc. Cosmet. Chem, 28, 457-479 (August 1977) Prediction of optimum O/W emulsification via solubilization measurements T. J. LIN 628 Enchanted Way, Pacific Palisades, CA 902 72 HARUKI KURIHARA and HIDEAKI OHTA Takasago Perfumery Co., Ltd., Tokyo Central P. O. Box 1033, Tokyo, Japan. Received August 6, 1976. Presented Ninth IFSCC Congress, June 1976, Boston, MA. Synopsis In the course of investigating the effects of SURFACTANT LOCATION on O/W EMULSIFICATION, it was discovered that there existed a useful correlation between the maximum amount of aqueous phase that could be solubilized in the oil phase containing the emulsifier and the average droplet size of the emulsion subsequently formed. Experiments were carried out with liquid hydrocarbons and many other oils frequently used in cosmetic emulsions. The emulsifiers used included various nonionic, anionic, cationic surfactants, and their mixtures. Analysis of the solubilization measurements and microphotographically obtained emul- sion droplet size distribution data clearly indicated that the point of optimum O/W emulsification, i.e., the point where the finest O/W emulsion was formed in emulsifying with a series of surfactant mixture, cor- responded to the point of maximum solubilization provided that the latter fell in a region where O/W emul- sion formation was possible. In some systems studied, the maximum solubilization points were found in the region where only W/O emulsions could be formed under }he experimental conditions. In such a case, the optimum O/W emulsions were generally found near the W/O-O/W transition point. The correlation held quite well in spite of the differences in the type and ionic nature of the surfactants employed. INTRODUCTION In spite of the recent advances in colloid and surface chemistry, the technique of emul- sion formulation and manufacturing remains very much an art. Although, it has been 27 years since Griffin (1,2) first proposed the HLB (hydrophile-lipophile balance) method, the selection of an emulsifier system for a practical cosmetic emulsion still re- quires a tedious trial-and-error procedure. This is chiefly due to the extremely complex nature of emulsions, which often defies systematic scientific treatment. Fundamentally, HLB is a very useful system in classifying surfactants according to their hydrophilic/lipophilic characteristics. It is, also, recommended as a tool for selecting efficient emulsifiers for preparing emulsions nevertheless, in this respect, there are many shortcomings which hinder its practical applications. First of all, to use HLB method for emulsification, one needs not only the "HLB" values of the surfactants but also the "required HLB" values of all the oil phase components to be used in the emulsion. Unlike the HLB values of the surfactants, the 457
458 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS available data on required HLB values are quite limited and the published values are often conflicting. This is partly due to the fact that there is still a lack of a reliable and accurate method to determine the required HLB value of an oil (3). Second, even for the oils for which required HLB values are available, the HLB method provides only a very rough guide in finding a right emulsifier combination. Even if a formulator knows that he requires a certain HLB to emulsify a given oil, he still needs to carry out many trial-and-error emulsifications, using combinations of surfactants with different chemical types before finding a suitable combination for his practical purpose. The HLB method provides no further guidance in this respect. Third, the HLB method assumes that like HLB values of the surfactants,.required HLB values of oils are also linearly additive. This linear relationship has been found to be questionable in many oil mixtures (4). Furthermore, Griffin's HLB method assumes that both the HLB value of a surfactant and the required HLB value of an oil are constants independent of other parameters. This assumption makes the HLB method quite simple to use however, it also makes the method less precise and sometimes unreliable, since many other factors such as aqueous phase additives, surfactant concentration, phase volume of the oil, emulsification temperature, or even the preparative method can influence the hydrophilic/lipophilic characteristics of emul- sions (5,6,7). Furthermore, the HLB method works fairly well if one uses only ethoxylated nonionic surfactants to emulsify hydrocarbons. It often fails to work satisfactorily, however, in many practical cosmetic emulsions containing a complex mixture of oils, fatty ma- terials, polar substances, and various surfactants. Clearly, there is a need for a better system to aid emulsion formulators to select the most efficient emulsifier combination from the great number of commercial surfactants available today. During the course of investigating the effects of surfactant location and migration on emulsion properties, it was discovered that there appeared to be a correlation between the maximum amount of the aqueous phase which could be solubilized in the oil phase containing the emulsifiers, and the average droplet size of the emulsion subsequently formed. For a given pair of surfactants, one relatively hydrophilic and the other rela- tively lipophilic, the most efficient emulsifier combination was generally found at the point where there was the greatest amount of solubilization. After testing over 100 systems with varying oils, surfactants, and other additives, it is believed that this cor- relation can be very useful in aiding emulsion formulation and to minimize the need for a trial-and-error procedure. EXPERIMENTAL For low-speed emulsification, emulsions were prepared by first dispersing the suffactants in the oil phase using a mixer. Sixty-five g of aqueous phase was first placed in a 200 ml beaker, and 35 g surfactant-oil mixture was carefully placed on the top. A 2 x 6 cm flat blade paddle mixer, set 5 mm above the bottom of the beaker, was turned on immediately to start emulsification. In most experiments, the emulsification was done at room temperature (21 ø +• IøC), and the emulsions were mixed for 3 min at exactly 150 rpm before droplet size measurements. For high-speed emulsification, a rotary homomixer was used. All emulsification operations were carefully done to assure good reproducibility.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)