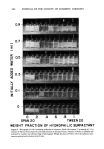
OPTIMUM O/W EMULSIFICATION 477 the equivalent solubilized state of Z with the method of emulsification employed. With more added water, the hydrophilic surfactant soon migrates to the aqueous phase mak- ing it impossible for mechanism A to function. The third requirement of mechanism A concerns phase inversion. In some systems, poor matching of the solubilization peak and the point of optimum emulsification were believed to be due to the phase inversion effect. As illustrated in Fig. 13 (A), phase inversion has no effect on the correlation when its boundary, indicated by the dashed line, is located on the left-hand side of the solubilization peak S. The optimum emulsification point E generally coincides with the peak in such a case. If the phase inversion boundary should fall on the peak as illustrated by Fig. 13 (B), the optimum emulsification point E generally shifts slightly to the right since an inverted W/O emul- sion or mixed emulsion is formed at S. If the peak S occurs within the W/O region and the amount Of solubilization is very small in the O/W region as illustrated by Fig. 13 (C), no optimum emulsification point exists as all O/W emulsions made have large droplet sizes. It should be noted that phase inversion of an emulsion is dependent not only on the hy- drophilic/lipophilic nature of the surfactants but also strongly on other variables such as internal phase volume, surfactant location, and the method of emulsion preparation (7, 10). Hence, the phase-inversion boundary can shift depending not only on the formulation, but also on the process variables such as the rate of addition of one phase to the other phase, degree of agitation, emulsification temperature, etc. In 1964, the PIT (phase inversion temperature) method of selecting emulsifiers was suggested (11, 12) as an alternative to the HLB method. The PIT of an emulsion is de- pendent not only on the type of surfactants and oils, but also on other parameters such as phase volume, surfactant concentration, or the presence of salts. With regard to this, the PITs are said to provide more accurate information than the HLB, required HLB values which do not account for these effects. However, in practice, the PIT system, like HLB, also has shortcomings. First, systems containing anionic or cationic surfactants do not exhibit PIT and, therefore, the method would not apply. Second, since PIT is dependent on so many parameters, it is more complicated to apply it in a practical system than the HLB method. Finally, the PIT system is also only good as a rough guide, since it merely tells the formulator that he should not use combinations having PITs too close to the temperature at which the product is to be used or stored. CONCLUSIONS The obvious value of the solubilization-emulsification correlation here is its application in selecting emulsifiers for product development work. Since a solubilization measure- ment is relatively simple and the results are reproducible, it provides a quick way to de- termine the point of optimum emulsification. It can be also used to determine the ef- fects of oil additives on emulsification, since the correlation holds not only for nonpolar oils, but also for many polar oils and their mixtures. The solubilization and phase inversion data can also be very useful in process develop- ment work for emulsion products. They can be helpful in finding the best manufactur- ing method and also in avoiding manufacturing troubles.
478 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS For example, solubilization data obtained at various temperatures are useful in finding an optimum emulsification temperature. If the emulsification is done by the surfactant- in-oil method, it is well to study the oil phase containing various amounts of solubilized water at various temperatures. As it was pointed out earlier, the oil-surfactant mixture containing some water may not be stable and a separation into surfactant-rich and surfactant-poor phases can result in an emulsion with extremely nonuniform droplet size distribution. Therefore, consideration as to when and how rapidly to add the aqueous phase to the oil phase may become a very important factor in preventing manufacturing difficulties. The method of solubilization measurements used in this w•ork relied upon visual observations and the solubilization limit was defined as the point beyond which a permanent turbidity would develop upon addition of more water. This method is very simple to use, but it does have some disadvantages. In some systems, turbidity does not develop sharply, resulting in a difficulty in determining the endpoint. Another problem is that if the mixture is not completely transparent at the temperature of measure- ments, it would be very difficult or impossible to judge the endpoint. This would rule out the application of the method to oil mixtures containing two immiscible oils (e.g., mineral oil and silicone fluids) or to the mixture containing a solid suspension. However, it is believed that such difficulties can be overcome by using other means of determining solubilization. One promising method is the use of vapor pressure measurements which has been successfully used in measuring solubilization of water in nonaqueous systems (14, 15). The vapor pressure of an oil containing solubilized water generally shows an increase with increasing amount of solubilized water until the maximum point is reached. Since the method is not dependent upon a visual observa- tion, the previously mentioned difficulties would not occur. Unlike emulsion stability which is extremely difficult to define, solubilization is a bet- ter-defined phenomenon and can be related to the physical and chemical properties of the materials involved. Therefore, it is believed •hat the correlation presented here can be a very valuable basis for developing a useful tool for emulsifier selection, which is more accurate and reliable than other existing methods. ACKNOWLEDGMENT The authors gratefully acknowledge many valuable suggestions given by Dr. T. Moroe of Takasago Perfumery Co., Ltd. REFERENCES (1) W. C. Griffin, Classification of surface-active agents by"HLB", J. Soc. Cosmet. Chem., 1, 311-26 (1949). (2) W. C. Griffin, Calculation of HLB values of nonionic surfactants, J. Soc. Cosmet. Chem., 5, 249-55 (1954). (3) W. C. Griffin, H. J. Ranauto, and A.D. Adams, Further studies on emulsion systems, Amer. Perfum, Cosmet., 81, 31-42 (1966). (4) N. Ohba, Required hydrophile-lipophile balance values of the oil mixture, Bull. Chem. Soc. Jap., 35• 1021 (1962). (5) P. Sherman, Emulsion Science, Academic Press, New York, 1968, Pp. 140-53.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)