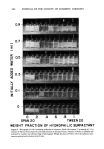
OPTIMUM O/W EMULSIFICATION 469 9 8 z I,, I I I i • ,•1 ß ß •$ _ O---MEAN DROPLET SIZE A--- SOLUBILIZATION LIMIT ....... MINERAL OIL ONLY o o SPAN 20 WEIGHT I 8.6 .2 .4 FRACTION OF 10 HLB i LAURYL ALCOHOL ß ..' .6 .8 HYDROPHILIC 15 i,i 10n.' z o 1 TWE E N 20 $URFACTANT ' I 16.7 Figure 8. Shifting of optimum emulsification peak by addition of lauryl alcohol. (Emulsions contain 30 per cent oil phase, 65 per cent deionized water, and 5 per cent surfactant mixtures. Surfactant mixtures consist of hydrophilic Tween 20 and lipophilic Span 20 at ratios and corresponding HLB values indicated by abscissa. Dotted lines represent data for pure mineral oil systems. Solid lines represent data for oil mixture consisting of 8 parts mineral oil and 2 parts lauryl alcohol)
470 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS use the literature HLB value of 1.15' to repeat the above calculations, one would end up with a result requiring no Arlacel 80 but a very large amount, 354 g, ofTween 80 for the optimum emulsification. This means that by merely altering the functional con- cept of a component, one can come up with a vastly different surfactant requirement. This is a serious problem, since there are so many common cosmetic ingredients like cetyl alcohol, which can be regarded either as oils or auxiliary emulsifiers. In reality, these materials probably function partially as an emulsified oil and partially as surfactant in practical emulsions. In the HLB system, however, one is forced to regard them either as a surfactant to emulsify or an oil to be emulsified, but unfortunately, these 2 alternatives do not lead to a consistent result. This is probably one of the main reasons why the HLB method does not work well for many practical emulsions containing polar substances. The fact that the addition of a polar oil to a nonpolar oil often affects the required HLB of the system far more than that can be predicted from the simple additive rule can be demonstrated by considering the required HLB of lauryl alcohol which is given in the literature as 14 (8). Thus, a 20/80 mixture of lauryl alcohol/mineral oil has a required HLB value of 10.8 using the linear additivity rule, taking the required HLB value of mineral oil as 10. This means that one should expect no more than 1 unit shift of the re- quired HLB by substituting 20 per cent of mineral oil with lauryl alcohol. However, our emulsification experiments with mixed oils indicated that the shift was considerably greater than one unit. In Fig. 8, the dotted lines indicate the solubilization and emulsification curves for pure mineral oil emulsions prepared with Tween 20 and Span 20. The solid lines on the same figure present the results of substituting 20 per cent of mineral oil with lauryl alcohol. It is clear that the optimum emulsification point shifted about 2.4 HLB units after adding lauryl alcohol to the nonpolar mineral oil. The maximum solubilization point also shifted to the right by the same proportion indicat- ing the reliability of the solubility measurement as a means to predict optimum emulsification. Similar experiments with other polar oils such as oleyl alcohol or oleic acid indicate that the solubilization method can reliably predict the optimum emulsification point even in cases where the HLB system failed. RELATIONSHIP WITH EMULSIFICATION MECHANISMS In our previous work, we attempted to explain a dramatic difference in O/W emulsification efficiency due to the initial locations of the hydrophilic surfactants by proposing 2 separate mechanisms in emulsification (9). According to our hypothesis based on experimental evidences, mechanism A, which is the mechanism that produces the finer emulsion, can control emulsification when a relatively hydrophilic surfactant is initially disssolved in the oil phase. As water is added to this oil-surfactant mixture to start emulsification, the water is first solubilized in the oil phase and a W/O emulsion is formed. As more water is added, the hydrophilic surfactant starts to migrate to the aqueous phase resulting in an emulsion phase inversion to form an O/W emulsion. A short-lived double emulsion of (W/O)/W type may be formed during the transition stage. The phase inversion results in a production of emulsion with a fine droplet size. *The experimental value of the HLB of cetyl alcohol is given as 1.0 while the calculated HLB according to Davies' group number is 1.3 (5). The average value of 1.15 is used in this calculation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)