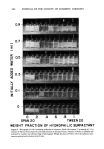
OPTIMUM O/W EMULSIFICATION 471 (B) Figure 9. Microphotographs showing improvement of emulsification by adding 2 per cent water to make the oil phase homogeneous. (Emulsions contain 30 per cent mineral oil, 65 per cent deionized water, and 5 per cent surfactant mixtures. Surfactant mixtures consist of Tween 80 and Arlacel 80 at 80/20 ratio.): (A) emulsion made with nonhomogeneous oil phase (x 1200) (B) emulsion made with homogeneous oil phase containing 2 per cent water (x 1200) When the surfactant is placed in the aqueous phase, phase inversion does not take place, and the oil droplets are broken up by the mechanical shear provided by the mixer. This was referred to as mechanism B, and unless a relatively high speed is used to process the emulsion, the resulting emulsion will have a much larger average droplet size than the same formulation prepared via mechanism A.
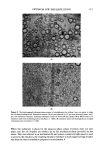
472 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS -, • ...i :' ?' ' ... :5::" ß •' . .•-•-.,.: . : , ,.. .= .• • .... .. •.• .... . .: .• . . .., -' -• ..•.. • . 5. -., . Figure 10. Microphotographs showing mineral oil emulsions with 2 distinct droplet size distributions. (Emulsion contains 30 per cent mineral oil, 65 per cent &ionized water, and 5 per cent surfactant mixtures. Surfactant mixtures consist of POE (10) oleyl ether and POE (2) oleyl ether at 40/60 ratio) (x 1200) There are 3 conditions which must be fulfilled before Mechanism A can operate. They are as follows: (1) the surfactants must be soluble in the oil phase in which it is initially placed (2) the surfactants in the oil phase must solubilize or emulsify a part of the aqueous phase and (3) a phase inversion must take place to form an O/W emulsion. Further work using various oils and nonionic, anionic, and cationic surfactants resulted in no data contradictory to the proposed hypothesis. A careful analysis of the experi- mental data indicates that wherever any of the above 3 conditions were promoted, the emulsification efficiency improved. On the other hand, whenever a factor was in- troduced to hinder any of these conditions, the emulsification efficiency often dra- matically decreased. With regards to the first requirement of surfactant solubility in the oil phase, for example, an oil phase containing 14.3 per cent Tween 80/Arlacel 80 mixture at 80/20 ratio does not form a homogeneous phase. Upon standing, a surfactant-rich phase would separate from the mixture and settle to the bottom. The emulsion, prepared by quickly adding water to such a mixture with a moderate mixing, had coarse droplets and was unstable (Fig. 9(A)). However, by initially dispersing about 2 per cent of water in the oil phase, the mixture became homogeneous and the emulsion prepared improved remarkably as can be seen in Fig. 9(B). The small amount of the water added to the above mentioned mixture apparently had a significant effect on the miceliar structure in improving the solubility of the surfactant in the oil phase. Sometimes a similar effect could be achieved by adding a small amount of polar oils such as oleic acid. The result is also a definite improvement of emulsifica- tion efficiency.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)