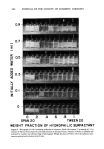
COSMETIC PROTEIN HYDROLYSATES 449 Sephadex Gels Sephadex G-10 G-15 G-25 G-75 G-200 METHODS Gel Filtration Cosmetic grade protein hydrolysates were thought to have average molecular weights of between 1,000 and 10,000. With this range, Sephadex G-15 or G-25 would be the gel of choice for the separation of these peptide molecules. Chromatographic columns were prepared by packing 1.5 x 100 cm columns with gel previously swollen in 0.25 M NaC1. These columns were equilibrated with the saline. Protein hydrolysate samples were dissolved in the equilibration solution at a concentration of 180 mg/ml, and 3 ml of this solution was applied to the top of the column. The material was eluted at the rate of 0.25 ml/min. The presence of polypeptides was detected by measuring the absorbance at 280 nm using a continuous flow spectrophotometer. The curves generated showed that all of the sample was eluted in the void volume (above in the upper exclusion limit) from the Sephadex G-15 column, and some of the sample was eluted in the void volume from the Sephadex G-25 column. These gels, therefore, were not the appropriate ones for the separation of the cosmetic grade protein hydrolysates being investigated. After the initial chromatographic runs were completed, Sephadex G-75 and G-200 columns were packed in the same manner as the G-15 and G-25 columns. From the absorbance graphs of these runs, it was determined that G-75 was the most useful in determining the molecular weight distribution of the hydrolysates being studied. The 3 commercially available cosmetic grade proteins described above were studied using the Sephadex G-75 column. The peptides were eluted with 0.25 M NaC1 at a rate of 0.15 ml/min and detected with absorbance measurements at 280 nm. To determine the molecular weight relationship to elution volume, the Sephadex G-75 column was calibrated. First, the void volume was determined by eluting Blue Dextran the elution volume is equal to the void volume. The total volume was calculated from the geometry of the column. A calibration curve was generated by eluting proteins of known molecular weights from the same Sephadex G-75 column and under the same conditions used in the experimental run. Protein Molecular Weight Aldolase 158,000 Ovalbumin 45,000 Chymotrypsinoge n 25,000 Ribonuclease A 13,700
450 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The elution volumes of the proteins shown in the above chart were plotted against the logarithm of the molecular weight. PROTEIN SUBSTANTIViTY OF MOLECULAR WEIGHT FRACTIONS Evaluation of the 3 unfractionated cosmetic grade proteins on hair showed that the enzyme digested hydrolysate was the most substantive. For this reason, it was selected as the protein for study of the substantivity properties of its various fractions. A large G-75 Sephadex column (5.0 x 100 cm) was used, since much greater quantities of ma- terial than could be obtained from the small column were required to conduct the substantivity measurements on the various fractions. The sample of the enzyme hydrolyzed material, 50 ml of an 18 per cent solution in 0.25 M NaCI, was applied to the bottom of the column and eluted at a rate of 0.5 ml/min. After 4 h, the column was inverted and the flow allowed to proceed as a descending chromatograph. This procedure insures a straight horizontal front. Fractions were collected every 15 min. These fractions were then pooled into 4 main fractions: (1) greater than molecular weight 30,000 (2) 30,000-5,000 (3) 5,000- 1,000 and (4) less than 1,000. It was necessary to desalt each of the fractions, because each was dissolved in 0.25 M NaCI. Desairing was accomplished on a Sephadex G-10 column. Each of the fractions was first freeze-dried, redissolved in 10 ml of water, ap- plied to the column, and eluted with water. The solid material was used to prepare 5 per cent solutions of each fraction. The relationship of molecular weight to substantivity to bleached and bleached-waved hair was then investigated. Hair swatches of each type of hair were prepared and each was then treated with one of the 5 per cent solutions. A water control and an hydroxyproline treated swatch were also included in the analysis. The treatment consisted of soaking for 10 min, blotting off the excess, and rinsing for 30 sec in warm running tap water. To determine the amount of protein sorbed on the hair, the swatches were hydrolyzed with barium hydroxide, and the hair hydrolysate analyzed for hydroxyproline, the amino acid found in collagen protein but not in hair. In addition, the amount of hydroxyproline in each fraction was determined in order to correlate per cent hydroxyproline to per cent protein (6). Ultracentrif ugation Two of the fractions separated by the Sephadex G-75 column were prepared for ultracentrifugation on a Beckman Ultracentrifuge* by diluting to 2 mg/ml in 0.25 M NaC1. This solution was placed in 1 side of a filled-Epon double sector synthetic boundary center piece,* and 0.25 M NaCI was placed in the other side. The cell with sapphire windows was placed in an AN-D rotor, and the run started. The conventional sedimentary equilibrium method of determining molecular weight was used. The ex- perimental set-up for the 2 runs was as follows. *Beckman Instruments Inc., Palo Alto, CA.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)