OPTIMUM O/W EMULSIFICATION 463 ethylene oxide in the surfactant. The average droplet sizes of the emulsions obtained from the microphotographic measurements were also indicated in Fig. 1. It is clearly seen from Fig. 1 that a maximum solubilization in this series of surfactants was obtained when the 5 mol ethylene oxide adduct was used and that this surfactant also gave an emulsion with finest mean droplet size. Figure 2 shows a similar correlation obtained with various mixtures of 2 and 7 mole ethylene oxide adducts of oleyl ether. Here again, a good correlation between the maximum solubilization point and the minimum droplet size was obtained. The HLB of the surfactant blend at the optimum point was 9.7 calculated from the supplier's ex- perimentally determined HLBs of the 2 and 7 mole adducts, which were given as 7.7 and 10.7, respectively.* This value is fairly close to the literature value of the required HLB ofparaffinic mineral oil which is about 10. Figure 3 shows another example of mineral oil emulsion emulsified with combinations of Tween 20 and Span 20. Here again, a good correlation was obtained at Tween 20/Span 20 ratio of about 40/60. In most systems, the solubilization data were obtained through drop-by-drop addition of water into the oil-surfactant mixture with mixing as described earlier. In a few systems, the solubilization curves were carefully studied by shaking varying amounts of water into vials containing the oii-surfactant mixtures and equilibrating the systems before making observations. Figure 4 shows a photograph taken shortly after the preparation of the representative vials used in the Tween 20- Span 20 system. TESTING OF THE CORRELATION In order to test the validity of the correlation, many different types of surfactants, oils, and other additives were employed for solubilization measurements and corresponding emulsification experiments. In addition to nonionic surfactants, anionic and cationic surfactants and their combina- tions were also tested. The data for an anionic-nonionic system using 96 per cent active sodium dioctyl sulfosuccinate (SDOS)? and sorbitan monooleate (Arlacel 80)• are shown in Figure 5. A fairly good correlation is apparent for this mixed-surfactant system as both the solubilization peak and the droplet size minimum are located around 0.6 weight fraction. It is noted that below approximately 40 per cent of SDOS, the emulsification became extremely poor due to the phase inversion. The point of phase inversion is indicated by the vertical dashed line. In most of the systems tested, the phase inversion occurred at points near the left end of the diagram and sufficiently removed from the solubilization peaks so that practically no effect on the correlation was observed. However, in some systems, the phase inversion boundary fell on or near the peak as illustrated by Figure 6 which em- *These are the values given by Nikko Chemicals Co., Ltd. The HLB values of similar surfactants given by ICI United States Inc., are 4.9 and 10.7, respectively. This would give the HLB at the optimum point as 8.7. ?Tokyo Kasei Kogyo Co., Ltd. of Tokyo, Japan. $Kao-Atlas Co., Ltd. of Tokyo, Japan.
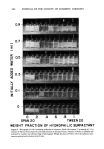
464 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS I.l.I .._l ß : 0.1 ' '" ':' .... :•'""""' ' '•' "" • 'i. ' ß ,.•. • o 0 .2 .4 .6 SPAN 2 0 TWEEN 20 WEIGHT FRACTION OF HYDROPHILIC SURFACTANT Figure 4. Photograph of vials containing surfactant-oil mixtures. (Each vial contains 15 g mineral oil, 2.5 g mixture of Tween 20 and Span 20, and small amounts of deionized water. Amounts of water in milliliters are indicated by numbers on left-hand edge of photograph. Weight fractions of Tween 20 in the surfactant mix- tures are printed under bottom row of vials)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)