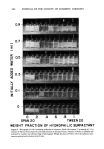
OPTIMUM O/W EMULSIFICATION 467 6 OIL: IPm o 5 6 • o z ß .- 0 E 5 • P- Li..I z 3 •" o • 3 o ,.n 2 •:) z .J 2 0 1 1 0--- MEAN DROPLET $1ZE• SOLUBILIZATION LIMIT, TURBI AREA 0 o 0 .2 .4 .6 .8 1 ARLACEL 80 TWEEN 80 WEIGHT FRACTION OF HYDROPHILIC $URFACTANT Figure 7. Solubilization-emulsion droplet size correlation for isopropyl myristate systems. (Emulsiomix- contain 30 per cent IPM, 65 per cent deionized water, and 5 per cent surfactant mixtures. Surfactant tures consist of hydrophilic, Tween 80, and lipophilic Arlace180 at ratios indicated by abscissa)
468 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ployed a cationic-nonionic surfactant combination. The surfactants used were lauryl dimethyl be nzyl ammonium chloride * and Arlacel 80. In this example, the point of op- timum emulsification does not precisely coincide with the solubilization peak, but is located somewhat to the right of the peak. This is clearly due to a phase inversion, from O/W to W/O, taking place near the solubilization peak. In addition to mineral oil, many other oils commonly used in cosmetics were tested. Figure 7 gives an example using isopropyl myristate (IPM) and nonionic surfactants. The phase diagram for this system is somewhat more complex due to the appearance of turbid areas under the solubilization curve. In this case, however, the turbid areas did not affect the correlation. In other more complex systems, as will be explained later, the turbid areas could shift the point of optimum emulsification. The use of the HLB method often does not work satisfactorily in m•tny emulsions containing polar oils. It is believed that a part of the problem is probably related to a flaw in the basic concept. The "HLB-required HLB" emulsifier selection method im- plicitly divides an emulsion into two parts: a surfactant or a surfactant mixture which is to emulsify, and an oil or oil mixture which is to be emulsified. In dealing with raw ma- terials for practical emulsions, however, it is not always possible to make such a clear distinction. For example, lanolin is generally regarded as an oil, but it can also serve as a low HLB emulsifier. Fatty alcohols or fatty acids are commonly used cosmetic ingredients for the oil phase, but they are surface active and can be adsorbed at the oil-water interface. If one considers them as oils to be emulsified, then their required HLB values must be used to calculate the required HLB of the entire oil phase. If one considers them as surfactants, then their HLB values must be included in the surfactant mixture. The problem is that one does not get a consistent result by interchanging the HLB, re- quired HLB values, indicating that there is an inherent inconsistency in this system. This can be best illustrated by considering the following example. Suppose that it is desired to emulsify an oil mixture consisting of 800 g mineral oil and 200 g cetyl alcohol, using a blend ofTween 80? and Arlacel 805. If one first regards cetyl alcohol as an oil, the "required HLB" of the oil mixture is cal- culated as follows (8): required HLB of mineral oil, paraffinic = t0 required HLB of cetyl alcohol = 15. Therefore, required HLB of the oil mixture = 0.8 (t0) + 0.2 (15) = 11. Taking the HLBs of Tween 80 and Arlacel 80 as 15 and 4.3, respectively (8), one readily determines the optimum ratio of Tween 80/Arlacel 80 for this mixture to be 1.67. If the total amount of the nonionic surfactants is to be tOO g, one needs 62.6 g Tween 80 and 37.4 g of Arlacel 80 to emulsify the cetyl alcohol-mineral oil mixture ac- cording to the HLB method. Alternatively, if one should regard the 200 g cetyl alcohol as a low HLB emulsifier and *Nissan Cation M2-100 by Nippon Oils and Fats Co., Ltd., Tokyo,Japan. q-Polyoxyethylene (20) sorbitan monooleate, ICI United States, Inc. *Sorbitan monooleate, ICI United States, Inc.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)