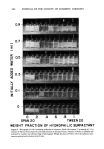
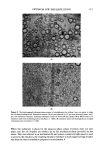
OPTIMUM O/W EMULSIFICATION 459 Emulsion droplet size distribution was determined from the Polaroid* pictures taken through an optical microscope. The amount of aqueous solubilization was determined by adding the aqueous phase, drop by drop, into the oil phase containing the surfactant while constantly mixing with a magnetic stirrer. The first sign of permanent turbidity was taken as the end point and the total amount of the aqueous phase added was recorded. In cases where a complete solubilization phase diagram was desired, the oil phase was placed in a large number of capped vials and shaken with varying amounts of water. After equilibration, the vials were observed for any sign of separation or turbidity and a phase diagram was constructed. RESULTS AND DISCUSSION CORRELATION OF EMULSIFICATION EFFICIENCY WITH SOLUBILtZATION There were 2 main purposes in this investigation. The first was to determine the validity and scope of the correlation between the efficiency of emtfisification and the maximum amount of aqueous solubilization by the oil phase containing the surfactants. The second aim was to investigate the fundamental role of the solubilization process and its relationship with emulsification. In this work, emulsification efficiency refers to the efficiency with which a surfactant or a mixture of surfactants emulsify the oil phase to form an emulsion without the use of high-shear equipment. A more efficient surfactant is defined as one which produces an emulsion with a finer average droplet size than a less efficient one under the same degree of mechanical agitation. Generally speaking, an emulsion with a smaller average droplet size is more stable than one with a larger droplet size. However, for this inves- tigation, the emulsification efficiency was directly expressed in terms of droplet size distribution immediately after emulsification rather than the emulsion stability. This choice was made in order to avoid possible confusion in interpreting the data, since emulsion stability is not only a function of droplet size, but also of many other parameters such as the viscosity of the external phase which is often influenced by the presence of the surfactants. In preparing most emulsions, a moderate mixing speed (150 rpm) was used. The use of an excessively high mixing speed wotfid promote the break-up of droplets caused by mechanical shear and obscure the real effects of the emulsifiers. The correlation appears to hold both for O/W emulsions prepared with single surfactants and also the emulsions made with combinations of two surfactants, one rela- tively hydrophilic, and the other relatively lipophilic. Figure 1 is an example of the data obtained with a series of ethoxylated nonylphenols with ethylene oxide ranging from 2 to 20 moles. Strictly speaking, these are not single surfactants, since they are commercial materials which are expected to have a wide ethylene oxide distribution range. Solubilization limit was defined as the maximum amount of water in milliliters which could be solubilized into a total of 100 g of oil- surfactant mixture. The abscissa represents the average weight percentage of the *Polaroid Corp., Cambridge, MA.
460 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS z 3 , O-- MEAN DROPLET SIZE i m /X SOLUBILI ZATION LIMIT (5) 7.5) lO) 18) 20) 30 0 ' ' , (...__•) m m I m m , 0 0 2 0 4 0 60 80 100 o o m o z WEIGHT % ETHYLENE OXIDE IN 5URFACTANT Figure 1. Solubilization-emulsion droplet size correlation for single surfactant systems. (Emulsion contain 30 per cent mineral oil, 65 per cent deionized water, and 5 per cent polyoxyethylene nonylphenyl ethers with per cent ethylene oxide corresponding to abscissa. Number in parenthesis indicates actual mole number of E. O. in each surfactant)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)