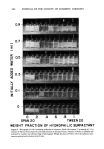
ANTIPERSPIRANT EFFICACY 445 Two different type alcohol soluble products, which are applicable for anhydrous systems, are aluminum chlorohydrate-propylene glycol complex (A) and alcohol solu- ble aluminum chlorohydrate (B). Table III shows per cent sweat reduction values for these 2 materials at identical aluminum concentrations. The efficacy of B is greater than A. The difference in results may be a function of the water content of the active in- gredient and, hence, the ethanol-water balance of the formulation. For example, the 20 per cent alcoholic aluminum chlorohydrate system contains ca. 4 per cent water, whereas the 25 per cent aluminum chlorohydrate-propylene glycol complex system contains a maximum 1 per cent water. It is possible that small amounts of water are necessary to catalyze antiperspirant activity of the metal salt. We plan to study thoroughly the relationship, if any, that exists between ethanol:water ratios and efficacy for a variety of alcohol soluble antiperspirants. MULTI-INGREDIENT FORMULATIONS In the evolution of antiperspirant formulation technology, combination systems of 2 or more active ingredients have recently generated much interest. Today, many cosmetic chemists prefer 2 active ingredients in their formulation instead of one component systems. For example, many formulators use aluminum chlo- rohydrate-AICla combinations. One reason for interest in these systems is the belief that more acidic products (e.g., aluminum chlorohydrate + AICh) have superior efficacy. There are hypotheses which correlate efficacy with pH. For example, the interaction of aluminum salts with skin protein is a function of pH (5). This type of reaction has been proposed as a possible mechanism for antiperspirant activity. Table IV. Per Cent Sweat Reduction for Aluminum Chloro- and Bromohydrate-A1Ca Combinations Concentration AI:C1 Ratio AI:Br Ratio Per Cent w/w 1:1 2:1 1:1 2:1 35 a 44 52 52 10 (23-48) b (33- 56) (41-62) (53- 63) 49 38 -- -- 20 (38-59) (27-48) -- -- apoint estimate per cent sweat inhibition. b95 per cent confidence limits. Table V Per Cent Sweat Reduction for AI-Zr Complexes at Different Ratios AI:Zr Ratio a Per Cent Sweat Reduction • 0.5: ! 45-54-66 -- 2.0:1 50-58-68 4.0: ! 48-59-70 6.0: ! 50-60-69 aAll at 10 per cent w/w. •Point estimate underlined. Outer points at 95 per cent confidence limits.
446 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS For example, the maximum reaction of aluminum chloride withskin protein occurs at a pH of 3.51, with the binding of aluminum falling off sharply on either side of the pH. At low pH levels, skin protein exhibits a decreased activity for aluminum ions due to the existence of its carboxyl groups predominantly as the undissociated --COOH species. At high pH levels, the carboxyl groups are ionized to the wCOO--state. Consequently, their interaction with aluminum would be expected to be enhanced. In light of the foregoing, it seems reasonable that sweat reduction mediated via the use of antiperspirants could be a function ofpH, assuming that the mechanism of such activity is controlled by the precipitation of skin protein with the basic aluminum species. Table IV compares efficacy results for aluminum chloro- and bromohydrate "types" with Al:halide ratios of 2:1 and 1:1. No significant difference between these lower and higher ratio products is evident for these aqueous formulations. It is, of course, possi- ble that the more acidic species are skin irritants and, therefore, act antagonistically (i.e., as "properspirants"), thereby attenuating the properties of the aluminum com- plex. In the search for new and effective antiperspirants, aluminum-zirconium combinations have aroused interest. We will only be concerned with nonaerosol aqueous formula- tions. Table V lists the efficacy for AI-Zr products, with AI:Zr ratios varying from 0.5:1 to 6:1. No significant differences in efficacy from product to product are evident. In general, it appears as if the effectiveness of these systems is comparable with 15 per cent aluminum chlorohydrate. It is believed, however, that these AI-Zr systems, once formulated, retain a higher proportion of their activity than aluminum systems that is to say, their effectiveness appears less influenced by the chemical environment represented by the formulation medium. SUMMARY To summarize, we believe that, based on our data, the efficacy of some antiperspirant materials peaks at a particular concentration rather than reaching a plateau. Reasons for this effect are unknown. Vehicle also plays a role in controlling efficacy. For example, anhydrous systems have a lower efficacy than aqueous or hydroalcoholic formulations. Our data regarding the relationship between efficacy and vehicle are limited. We plan to fill in this gap, in the near future, by studying the relationship between ingredient efficacy and vehicle as well as variations in AI:CI ratio and concentration. Finally, we have found that the efficacy of aluminum-zirconium complexes is independent of AI:Zr ratio. REFERENCES (1) P. A. Majors and J. E. Wild, The evaluation of antiperspirant efficacy--influence of certain variable, J. Soc. Cosmet. Chem., 25, 139-52 (1974). (2) M. W. Steed, Evaluation of antiperspirant preparations under normal conditions of use, J. Soc. Cosmet. Chem., 26, 17-28 (1975). (3) W. M. Wooding and P. Finkelstein, A critical comparison of two procedures for antiperspirant evalua- tion, J. Soc. Cosmet. Chem., 26, 255-75 (1975). (4) T. Govett and M. G. de Navarre, Aluminum chlorohydrate, new antiperspirant ingredient, Amer. Perfum., 49, 365-8 (1947). (5) I. Lyon and I. M. Klotz, The interaction of epidermal protein with aluminum salts, J. Pharm. Ass., 47, 509-12 (1958).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)