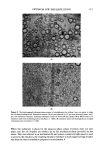
OPTIMUM O/W EMULSIFICATION 473 Another example is a mixture of POE (10) oleyl ether/POE (2) oleyl ether at 40/60 ratio in mineral oil.* If an emulsion is prepared by immediately emulsifying the oil- surfactant mixture right after dispersion, a reasonably fine emulsion is obtained. However, if the oil-surfactant is allowed to stand overnight, it would separate into two layers--the lower oil layer containing most of the surfactant and the top oil layer containing much less surfactant. If an emulsion is prepared with such a two-layered oil phase, the emulsion would contain fine droplets derived from the lower surfactant-rich layer and coarse droplets originated from the upper, surfactant-poor layer. A microphotograph of such an emulsion revealing 2 distinct droplet size distributions is shown in Fig. 10. The second requirement for the emulsification mechanism is related to the aqueouo solubilization by the oil phase. From the experimental results presented so far, it is quite apparent that water solubilization must be related to emulsification efficiency. Other factors being equal, a larger quantity of water solubilization appeared to favor formation of a finer emulsion, although, the quantity of solubilization by itself cannot be regarded as an absolute measure of emulsification efficiency. Although it is quite possible that a great water solubilization merely indicates the area of favorable condition for emulsification, there is also experimental evidence suggest- ing that solubilization is one of the necessary steps in the over-all emulsification process. In many systems studied, it was possible to improve emulsification by initially presolubilizing the water into the oil-surfactant mixture before emulsification. For example, a combination of 2- and 10-mole adducts ofoleyl ethers in mineral oil forms a fairly complex solubilization diagram as shown in Fig. 11. When an emulsion was pre- pared in the usual manner using an 80/20 surfactant mixture indicated by a letter "X" in the diagram, the droplets were very coarse and the emulsion unstable. Subsequently, the emulsification procedure was slightly modified by first dispersing and solubilizing a small amount (2.6 per cent) of water into the oil phase to bring the mixture to point "Z" in the diagram. The resulting emulsion was very stable and had a very fine droplet size as shown in the photograph in Fig. 12 (Z). Another similar presolubilized emulsification was carried out with a slightly reduced amount of initial water (2 per cent instad of 2.6 per cent) corresponding to point "Y" in the turbid area of Fig. 11. The result was an emulsion somewhat better than that of point X, but much inferior to the emulsion prepared at Z. Since all three emulsions, X, Y, and Z have an identical, final composition, it must be concluded that the presolubilization treatment and the amount of the presolubilized water were positively affecting the emulsification process. In dispersing the water into this oil-surfactant mixture, it was noted that the water was not solubilized instantly, but it required time and considerable amount of mixing work before complete solubiliza- tion was obtained. This would suggest that, perhaps, the rate of water solubilization too is an important factor in emulsification. It can be explained that when one starts emulsification from the point X by quickly adding water, even though, compositionwise, the mixture will pass points Y and Z, be- cause of the very slow rate of solubilization, it is not possible for the mixture X to reach *The total surfactant mixture was 14.3% in the oil phase.
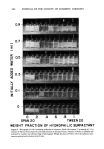
474 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 6 5 o o ---4 •3 z I I i ! i i i i 1 i O---MEAN DROPLET SIZE A--- SOLUBILIZATION LIMIT O---TURBID AREA z 15 lO 5 LU N LU n O Z x o o o .2 .• .6 .B 1 POE(2) OLEYL ETHER POE(10) OLEYL ETHER WEIGHT FRACTION OF HYDROPHILIC $URFACTANT Figure 11. Complex solubilization diagram (emulsions contain 30 per cent oil phase, 65 per cent deionized water, and 5 per cent surfactant mixtures. Surfactant mixtures consist of hydrophilic POE (10) oleyl ether and lipophilic POE (2) oleyl ether at ratios indicated by abscissa)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)