78 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table V Recovery Of 2-Pyridinethiol In Clear Shampoo mg/kg Added mg/kg Found % Recovery 53.7 52.8 97.2 54.5 53.7 98.5 56.5 56.2 99.5 53.3 52.5 98.5 53.6 52.8 98.6 53.3 53.0 99.4 x = 98.6 RSD = 0.83% If the reaction occurs further down the column 2MP will elute later and may interfere with following peaks. The solution to this problem is to flush the column with a strong solvent, e.g., acetonitrile, to assure that PDS from previous samples has been eluted. An analogous problem can occur during the analysis of either 2MP or PDS when the column is contaminated with strongly retained metal complexes of OM. Since these complexes will also react with PDS, their presence in significant amounts can cause high results for 2MP and low results for PDS. The solution to this problem is to decontaminate the column with injections of PDS which will react with the complexes. The PDS should then be completely eluted. Two other reactions occur which must be considered. These are the reactions of OMDS with 2MP, and OMDS with PDS. While these reactions have at present not been completely investigated, their analytical significance is that the standard addition method cannot be used to analyze either 2MP or PDS in the presence of significant amounts of OMDS. Thus the LC recovery data of 2MP and PDS had to be obtained by spiking blank shampoo samples which did not contain OMDS. This presents no particular problem for the LC analysis of any of these compounds since external standards are used however, the standard addition method is routinely used in polarography. Thus the polarographic analysis of low levels of 2MP and PDS in the presence of OMDS, i.e., in clear shampoos, would be inconvenient at best and of poor detectability. The polarographic analysis of PDS in the presence of OMDS is also precluded by the close proximity of their half-wave potentials. Another consequence of these reactions is that the equilibrium concentrations of 2MP and PDS in the presence of excess OMDS are low. Table VI Recovery Of 2,2'-Dithiobis-Pyridine In Clear Shampoo mg/kg Added mg/kg Found % Recovery 59.1 60.2 101.8 51.7 52.0 100.5 52.0 52.0 100.0 51.8 52.4 101.2 51.9 52.6 101.4 52.0 52,7 101.4 X = 101.1 RSD = 0.67%
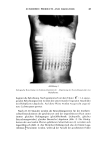
THE ANALYSIS OF OMADINE © BY LIQUID CHROMATOGRAPHY 79 PDS I I ! I ! I 012 34 ,_56 TIME (MIN) Figure 2. Separation of 2,2'-dithiobis-pyridine. Mobile phase: 50:50 acetonitrile/H20 containing 5 ml/1 of 40% tetrabutylammonium hydroxide solution, 4 ml/1 glacial acetic acid and 0.5 ml/l of saturated Na2EDTA. Flow rate: 1.5 ml/minute. Sensitivity: 0.01 AUFS. Reverse-phase liquid chromatography is a rapid and specific technique for the analysis of several compounds of interest in clear OMDS shampoos. This technique also makes possible the analysis of some compounds not accessible by polarography. In addition, the analysis time is much shorter for the LC method, particularly when several samples must be analyzed. REFERENCES (1) A. F. Krivis, E. S. Gazda, G. R. Supp and M. A. Robinson, Polarographic analysis of a series of metal ion 1-hydroxy-pyridine-2-thione systems, Anal. Chem., 35,966-968 (1963). (2) D. A. Csejka, S. T. Nakos and E. W. DuBord, Determination of sodium salt of 2-mercaptopyridine- N-oxide by differential pulse cathodic stripping voltammentry, Anal. Chem., 47,322-324 (1975). (3) Olin Corp. internal methods. (4) Olin Corp. internal methods. {• • ('•li,,ori_Vierh •ncl 1-1 I l•r•ec•7i•n Colorlmerrlc determination of sodium 2-Dvridinethiol 1-oxide with ferric ammonium sulfate, Anal. Chem., 48, 1001-1003 (1976).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)