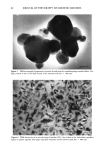
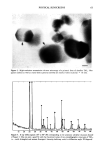
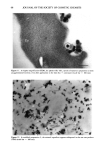
J. Soc. Cosmet. Chem., 47, 59-72 (March/April 1996) An electron microscopical approach to efficacy screening of physical sunscreens Received February 1996. Presented in part at the 6th European Society for Photobiology, Cambridge, UK, 1995, and at the Congr•s Annuel de la Socigtg de Recherche Dermatologique & 8th Journ&s de la Socigtg Fran•caise de Photo-Dermatologie, Clermont-Ferrand, France, 1995. JOSEPH HEMMERLE, PHILIPPE MSIKA, and ERIC GOORIS, Institut National de la Sant• et de la Recherche Mgdicale U.424, Place de l'H•pital, 67000 Strasbourg, France (J.H.), and Centre Recherche Dermo-Cosmgtique Pierre Fabre, 31322 Castanet Yolosan, France (P.M., E.G). Synopsis The aim of this study was to thoroughly investigate the spatial distribution of ultrafine titanium dioxide crystals in different physical sunscreen formulas as well as after their application onto human skin. The tested sun care products were realized by incorporation of two selected commercially available mineral blocking agents (TiO2) in two types of formulations. Due to the minute sizes of the oxide particles, the assessments were mainly carried out in scanning and transmission electron microscopy. Effectiveness of a physical sunscreen is directly related to the regular and uniform coating of the stratum corneurn with the mineral ingredient. Therefore, we systematically analyzed the microstructure of the TiO_, dispersion in the different creams. Above all, we focused our attention to the titanium dioxide deposit after topical appli- cations to the skin. The exposed results emphasize the importance of a proper selection of the raw material and demonstrate the role played by the cosmetic vehicle. INTRODUCTION It is now well established that excessive exposure to ultraviolet light (UV) is responsible for deleterious effects on skin. A survey of the literature reveals that much attention has been paid to UV-induced photoaging and photodamaging. While some authors inves- tigate the effect of ultraviolet B irradiation (1), others deal with the cellular defense mechanisms against longwave ultraviolet (UVA) radiation (2). It has been reported that the regular use of high sun-protection factor sunscreens could significantly reduce cutaneous neoplasia (3). Moreover, a randomized controlled trial revealed that, when sunscreens were regularly used over an Australian summer, sufficient sunlight was received through the sunscreen to allow adequate vitamin D production (4). These studies explain the widespread use of external photoprotection. The sun protection products are classified in two major categories: organic and mineral 59
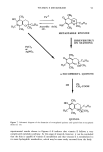
60 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS sunblocks. Both types of sunscreens aim to reduce the amount of UV light reaching the skin. Two particular difficulties are encountered when formulating physical sunblocks. First, one must select the most appropriate mineral raw material. Among the marketed mineral ingredients appear materials such as titanium dioxide, zinc oxide, iron oxide, and talc (5). These different sunscreen agents may exhibit different sizes and shapes. They also may undergo various surface treatments, already described for usual make-up cosmetic pigments (6). The second problem lies in the incorporation of the mineral into either an aqueous or an oil phase. For instance, a previous paper mentioned the possibility of an irreversible clumping of the mineral agent when used in a powder form (7). Nevertheless, the formulation must provide a good spreadability of the particles that have been worked into the formula. Ideally, a highly efficient physical sunscreen should provide a satisfactory protection against ultraviolet light, be aesthetically acceptable by appearing transparent when applied to the skin, and offer a silky feel. The purpose of this study was to examine the behavior pattern of ultrafine titanium dioxide crystals incorporated in various sunscreen formulations. Two types of TiO 2 were morphologically and crystallographically char- acterized. Three different sunblocks were realized and compared in electron microscopy. The mineral ingredient/cosmetic vehicle relationship as well as the spatial distribution of the metallic oxide particles onto the skin are of utmost interest for the improvement of physical sunscreens. Thus our investigations will undoubtedly contribute to a better knowledge of the reciprocal effects of the different factors. MATERIALS AND METHODS FORMULATION PRINCIPLES Sunscreen preparation 1. Acicular ultrafine titanium dioxide predispersed in mineral oil/ triglyceride (Tioveil MOTG, Tioxide Chemicals Ltd, UK) is encapsulated in oil droplets (H•liosides ©, Av&ne, a Pierre Fabre cosmetic patent) (8). Those microspheres are trapped in a ramified acrylate polymer base, thus yielding a steric stabilization of oil droplets in the aqueous gel. Consequently, the metallic oxides are hermetically isolated from the surrounding aqueous gel. This photoprotective microdispersion resembles an oil/water formula, but has the advantage to be formulated without any emulsifying surfactant. The TiO2 content in the final product = 6 wt %. The SPF is 6.9. Sunscreen-preparation 2. Prismatic ultrafine titanium dioxide powder (VP Titanium Di- oxide T805, Degussa, Germany) is added to the photoprotective microdispersion de- scribed above (sunscreen preparation 1). The prismatic oxides are confined in the oil microspheres (H•liosides©), thus separated from the acrylate gel. The TiO2 content in the final product = 6 wt %. The SPF is 8.7. Sunscreen preparation 3. The prismatic ultrafine TiO2 mentioned above (sunscreen prep- aration 2) is incorporated in a water/oil emulsion (a Pierre Fabre cosmetic patent). Optimal dispersion of the oxide particles is achieved by thorough grinding procedures within different specific esters and oils, thus breaking up the TiO 2 agglomerates. This total sunblock is formulated only with physical sunscreen agents. The TiO2 content in the finished product = 6 wt %. The SPF is 12.9.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)