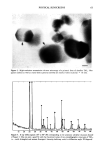
VITAMIN E METABOLISM 91 Identification •.0 4.•5 4. g' 3.5 2.5 2.0 t.• {3O 'cP$ xlo 280 130 .... gS• Oi3 ' ' • O•13g .... t5 130' ' ' Purity of [I:la]a-3,4-a-Tocopherui b [H312-3, 4- a-T 3.5 3.0' 2.• 1.5 0.5. 0t3" gO' ' 05 03 ' 10' 00 Figure 5. a: Chromatogram of ot-tocopherol and ot-tocopherol quinone in an HPLC assay with a UV detector. b: Chromatogram of radiolabeled ot-tocopherol with HPLC in line with a liquid scintillation counter. recovered with the same retention time as vitamin E, and so this tritium-labeled vitamin E was used for this study. With this technique, it was possible to exclude the interference caused by endogenous skin compounds that may leach out from the skin. This technique also allows for the metabolic pathway of radiolabeled ot-tocopherol during the skin permeation study to be traced. Figure 6 shows the chromatogram of a
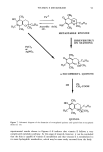
92 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 3.5 Figure 6. Chromatogram of a sample from receptor cell at 48 hours in vitamin E skin permeation study. sample from the receptor solution after 48 hours of skin permeation study. Total radioactivity was separated into three peaks. The peaks with retention times of 13 and 9 minutes were identified as ot-tocopherol and ot-tocopherol quinone, respectively. Considering our separation conditions, as shown in Table I, we can conclude that vitamin E was metabolized to a more hydrophilic metabolite, which may be an ot-to- copherol quinol as described in Figure 7 (13). The disappearance of peaks from time to time in the HPLC chromatogram, as observed in Figure 1, implies that there is some interaction between the dermis of the skin and vitamin E in receptor solution. This may be caused by back diffusion or further metabolism taking place by the enzyme leached from the dermis (14). METABOLISM OF VITAMIN E IN RECEPTOR SOLUTION WITHOUT CONTACTING THE SKIN To confirm that vitamin E is metabolized in the skin, a stability study of vitamin E in the receptor solution was performed. In Figure 8, the chromatograms clearly show that vitamin E was stable in the receptor solution for up to 24 hours. Vitamin E in contact with the skin showed very significant metabolic degradation, as shown in Figure 6. Although vitamin E undergoes some degradation in the receptor solution (first order degradation constant, K = 0.026 hr-1, data not shown) (15), there is no other peak except for vitamin E observed in UV analysis. In the liquid scintillation counter, most of the radioactivity was recovered as ot-tocopherol. This result clearly indicates that the major metabolism of vitamin E takes place in the skin and not in the receptor solution. In 1974, Shiratori observed the high and prolonged retention of radioactivity in the skin, comparing other tissue after intravenous injection of tritium-labeled vitamin E, and he suggested that skin may have an important storage, excretion, and metabolism function of vitamin E even though he couldn't provide the evidence (16). The present
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)