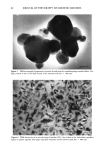
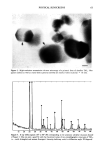
PHYSICAL SUNSCREENS 63 Figure 3. High-resolution transmission electron microscopy of a primatic form of ultrafine TiO 2 Hex- agonal outlines as well as crystal lattice patterns (arrows) are clearly evident (scale bar = lO nm). Figure 4. X-ray diffractogram (10 ø to 80 ø 20) corresponding to the prismatic ultrafine titanium dioxide of Figure 3 One can note a good fit with the theoretical values of two crystallographic structures of TiO 2, i.e., rutile (triangles) and anatase (lozenges). Intensity (arbitrary units) vs diffraction angle 20 (degrees).
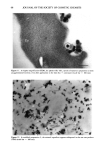
64 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS .w Figure 5. In SEM the H•liosides © appear as well-defined individual microspheres (scale bar = 100 •m). gram corresponding to the prismatic form of titanium dioxide observed in Figure 3. These XRD data match with both crystallographic forms of TiO 2 i.e., rutile (JCPDS file number 21-1276) and anatase LICPDS file number 21-1272). The large depth of field of SEM allows us to visualize the three-dimensional aspects of a particular cosmetic vehicle (H•liosides ©) used in sunscreen preparations 1 and 2. The mineral ingredients appear to be trapped in spherical structures, as shown in Figure 5. The spherical bodies (H•liosides ©, in preparations 1 and 2) have various sizes, with an average diameter of 2.58 •xm (standard deviation, cr = 1.05 •xm). Two selected TiO 2 raw materials (acicular and prismatic) were incorporated in those oil droplets (H•lio- sides©). When the acicular form of TiO2 was combined with the photoprotective microdisper- sion in sunscreen preparation 1, the crystallites appeared to be mainly confined to the periphery of the oil droplets. Some of those microspheres could even be observed after topical application to skin (Figure 6). Nevertheless, many areas showed a thin and regular layer of acicular TiO 2 crystallites lining the stratum corneum (Figure 7). When the prismatic TiO 2 powder was combined with the same photoprotective micro- dispersion in sunscreen preparation 2, it was again possible to visualize more or less spherical arrangements. However, in this case the crystals seemed evenly distributed in the oil droplets, thus occupying the whole room offered by the microspheres. This aspect was particularly evident along the laminar cells of hair cuticles (Figure 8). This different behavior of the mineral within the oil droplets (H•liosides ©) might be explained by the hydrophobic character of this prismatic titanium dioxide. After application of the photoprotective microdispersion onto skin, the burst micro-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)