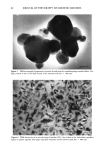
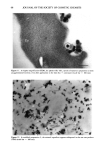
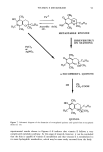
98 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS employed in cosmetic and skin care products for several years with great success. However, few reports on the deposition of glycolic acid and glycerol into skin after topical application have been published. Some in vitro transport studies were carried out to determine the permeation of glycolic acid (4) and glycerol (5) through the skin, but their accumulation in various strata of the skin following topical application was not determined. In the cosmetic industry, it is of interest to determine the extent to which the ingredients of the formulation accumulate in the stratum corneum and to determine whether or not they are transported into the living epidermis and beyond. The stratum corneum is the most important barrier to penetration through the skin and also acts as a reservoir for molecules applied to the skin (6). For some cosmetic ingre- dients such as glycerol, it is more important that they be retained in the stratum corneum in order to maintain skin moisturizing effects than to penetrate into the living strata. On the other hand, it may be necessary to transport compounds such as glycolic acid into the living skin strata in order for them to exert their therapeutic action resulting in improvement of the appearance of the skin. Thus, for optimum clinical effects it is necessary to design a formulation that will optimize delivery of actives to the appropriate skin strata. The purpose of this study is to investigate the kinetics of deposition of glycolic acid and glycerol in various strata of hairless mouse skin following topical in vivo application of a variety of formulations. MATERIALS AND METHODS MATERIALS Glycolic acid and propylene glycol (PG) were obtained from Sigma (St. Louis, MO). Glycerol was obtained from J. T. Baker Chemical Co. (Phillipsburg, NJ). The synthetic nonionic lipids, glyceryl dilaurate (GDL), glyceryl distearate (GDS), and polyoxyeth- ylene- 10-stearyl ether (POE- 10), as well as cholesterol (CH), were provided by IGI, Inc. (Little Falls, NJ). Tween 60, Arlacel 83, and Span 60 were purchased from CIC (Wilmington, DE). Paraffin soft was obtained from Sargent-Welch (Skokie, IL), and the mineral oil used was from Mallinckrodt (Paris, KY). The water used was double- distilled and deionized with a Millipore Milli-Q © system. 14C-Glycolic acid was ob- tained from Chemsyn Science Laboratories (Lenexa, KA). 3H-Glycerol was obtained from NEN (Boston, MA). METHODS Preparation of formulations Nonionic liposomal formulations. Two nonionic liposomal formulations, one containing glyceryl dilaurate / cholesterol / polyoxyethylene- 10-stearyl ether (Non- 1), and the other glyceryl distearate/cholesterol / polyoxyethylene-10-stearyl ether (Non-2), at a weight percent ratio of 57/15/28, were prepared by the syringe method. Appropriate amounts of the lipids were accurately weighed in a scintillation vial. The vial was then capped and heated with stirring at 75øC in a water bath to melt the lipids. The lipid melt was then drawn into a syringe preheated at 75øC and maintained at 75øC in a water bath. A second syringe containing appropriate amounts of 40 mg/ml glycolic acid or 25 mg/ml
DEPOSITION OF GLYCOLIC ACID AND GLYCEROL 99 glycerol in distilled water containing trace amounts of 14C-glycolic acid or 3H-glycerol was heated to 70øC in a water bath. The pH of the 40 mg/ml aqueous glycolic acid solution was measured to be 1.9. The two syringes were connected via a three-way Teflon stopcock, and the aqueous phase was rapidly injected into the lipid phase. The contents were then injected back and forth between the two syringes while being cooled under cold tap water. This process was continued till the mixture was at room tem- perature. The resulting liposomal suspensions were then examined by inverted light microscopy to assure the quality and integrity of the liposomal preparations. The total lipid concentration in all liposomal preparations was 50 mg/ml. The concentrations of glycolic acid and glycerol were 40 mg/ml and 25 mg/ml, respectively. The formulations were stored at 4øC overnight before use in the experiments. 30% PG/water solution. Glycolic acid or glycerol solutions were prepared using a solvent mixture containing 30% (w/w) propylene glycol in water. The glycolic acid and glycerol concentrations were 40 mg/ml and 25 mg/ml, respectively. A trace amount of •4C- glycolic acid or 3H-glycerol was also included in the solutions. Oil-in-water emulsions. Appropriate amounts of mineral oil, Tween 60, and Arlacel 60 were accurately weighed in a scintillation vial. The vial was then capped and heated with stirring at 80øC in a water bath to dissolve the lipids in the oil. The aqueous phase consisting of distilled water or aqueous solution of glycolic acid or glycerol with trace amounts of •4C-glycolic acid or 3H-glycerol was preheated to 80øC. The aqueous phase was then added to the vial containing the lipid/oil mixture and vigorously stirred with cooling under cold water. The concentrations ofTween 60 and Arlacel 60 were 3% and 2% (w/w), respectively. The ratio of the aqueous to oil phases was 80:20 (w/w). The concentrations of glycolic acid and glycerol in the emulsions were 40 mg/ml and 25 mg/ml, respectively. The formulations were stored at 4øC overnight before use in the experiments. Water-in-oil emulsions. Appropriate amounts of mineral oil, paraffin soft, Arlacel 83, and Arlacel 60 were accurately weighed in a scintillation vial. The vial was then capped and heated with stirring at 60øC in a water bath to melt and dissolve the lipids in the oil phase. The mixed oil phase was then accurately weighed in a syringe and maintained at 60øC. The aqueous phase, consisting of distilled water or aqueous solutions of glycolic acid or glycerol containing trace amounts of •4C-glycolic acid or 3H-glycerol, respec- tively, was accurately weighed in another syringe at room temperature. The two syringes were connected via a three-way Teflon stopcock, and the aqueous phase was rapidly injected into the lipid phase. The contents were then injected vigorously back and forth between the two syringes while being cooled under cold tap water. The concentrations of Arlacel 83 and Arlacel 60 were 3% and 1% (w/w), respectively. The oil phase consisted of 40 wt% mineral oil and 5 wt% paraffin soft. The glycolic acid and glycerol concentrations were 40 mg/ml and 25 mg/ml, respectively. The water-to-oil ratio in the emulsions was 45:55 (w/w). The emulsions were stored at 4øC overnight before use in the experiments. In vivo deposition studies Male hairless mice (SKH-hr-1, 50-60 days old, Charles River Breeding Labs) were initially anesthetized with sodium pentobarbital (60 mg/Kg, J.p.). Twenty-five •1 of the test formulation were applied to a 4-cm 2 area of the dorsal skin surface of the mouse
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)