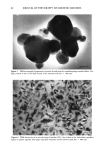
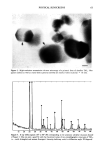
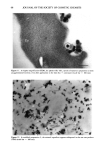
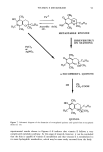
106 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table IV Distribution of Glycerol (expressed as percent of formulation applied + standard deviation) in Various Strata of Hairless Mouse Skin 16 Hours After Topical In Vitro Application of Various Formulations (n = 3-6) Formulation Compartment Non- 1 Non-2 30% PG/AQ Total donor 0.5 +-- 0.1 1.6 +-- 0.8 0.1 +-- 0.1 Total swabs 41.6 + 6.5 2.7 + 4.3 66.3 + 0.2 Strips 1,2 17.7 + 3.1 51.8 + 11.2 19.6 + 0.5 Total strips 19.6 + 5.1 52.7 + 10.5 19.7 + 0.6 Living skin strata 2.6 + 0.7 1.5 + 0.5 0.8 + 0.4 Receiver 26.0 + 5.0 38.2 + 8.3 7.5 + 2.0 Recovery 90.3 -+ 1.6 96.6 + 3.6 94.4 + 1.2 The apparent reversal of deposition efficiency of glycerol from Non-1 and Non-2 lipo- somal formulations may appear to contradict the mechanism of action of these formu- lations outlined for glycolic acid. However, it is important to reiterate that the critical factor in determining relative potency of Non-1 liposomal formulations in facilitating deposition into and across skin is the release of GDL and POE-10 from the liposomal bilayers. Any alteration in the experimental conditions, such as lowered temperature and occlusion or the presence of additives that alter this release process, will alter the overall deposition behavior of the Non-1 formulation. Thus, additives that delay release by delaying melting will lower deposition extent. The addition of a humectant such as glycerol to Non-1 liposomes delays melting of the lipid components by slowing down dehydration of the formulation, similar to the effects of lowered temperature or occlu- sion reported earlier (9). Non-2 liposomes, on the other hand, are not affected by the presence of glycerol since neither melting nor release of its components occurs at the skin surface temperature of 32øC. In essence, overall deposition eficiency from Non-1 lipo- somes is lowered in the presence of glycerol to the extent that Non-2 liposomes appear to be superior. The effects of inclusion of a humectant such as glycerol on the deposition of glycolic acid from Non-1 and Non-2 formulations further underscore the mechanism of action of these systems. A comparison of the results in Tables I and V indicates the effect of glycerol on the kinetics of deposition of glycolic acid from Non-1 and Non-2 liposomal formulations. The combined amounts of glycolic acid found in the living skin strata and urinary bladder are significantly lower at 4 h (p 0.05) and at 8 h (p 0.01) when glycerol is included in the Non-1 glycolic acid formulation, compared to the Non-1 glycolic acid formulation without glycerol. The addition of glycerol to Non-2 glycolic acid formulations does not affect glycolic acid deposition significantly (p 0.1 at all time points). The effect of glycerol on glycolic acid deposition from Non-1 liposomes is consistent with the delaying of melting and subsequent release of Non-1 components. The deposition from Non-2 liposomes is unaffected by glycerol since none of the Non-2 components melt or are released at a skin temperature of 32øC. It is also clear that the presence of glycerol in formulations may influence deposition character- istics of other active ingredients. In conclusion, the results of deposition studies of glycolic acid and glycerol from a variety of test formulations indicate clearly that nonionic liposomal formulations are more efficient in facilitating enhanced association of glycolic acid and glycerol with the
DEPOSITION OF GLYCOLIC ACID AND GLYCEROL 107 Table V Kinetics of Distribution of Glycolic Acid (expressed as percent of applied dose + standard deviation) in Various Strata of Hairless Mouse Skin After 1-Hour Topical In Vivo Application of Nonionic Liposomal Formulations Containing 2.5% Glycerol (n = 3) Stratum Living Time corneum Stratum skin Urinary (h) Swabs surface corneum strata excretion Recovery Non-1 liposomes with 2.5% glycerol 0 46.4 + 5.6 33.9 + 4.1 5.8 + 0.7 2.02 + 0.30 0.05 + 0.02 88.1 + 0.8 4 29.3 + 1.2 22.3 + 0.7 13.8 + 0.5 1.40 + 0.19 1.20 + 0.22 68.0 + 0.5 8 36.7 + 2.8 18.3 + 0.4 10.3 + 1.8 0.72 + 0.13 1.00 + 0.08 67.0 + 2.3 Non-2 liposomes with 2.5% glycerol 0 20.5 + 2.7 61.7 + 0.3 8.9 + 3.0 1.04 + 0.06 0.08 + 0.03 90.0 + 1.2 4 22.5 + 2.8 34.2 + 0.7 14.9 + 1.9 1.48 + 0.16 0.36 + 0.02 73.4 + 2.8 8 19.4 + 5.3 23.2 + 6.2 14.9 + 3.8 1.24 + 0.04 0.86 + 0.42 59.6 + 3.3 stratum corneum, in comparison with conventional vehicles. Further, the greater amounts of glycolic acid found in the living skin strata from these nonionic liposomal formulations indicate their utility in transporting actives to living tissue for therapeutic effects. The results also strongly suggest that of the two nonionic liposomes tested, Non-2 liposomes may allow increased retention of glycolic acid at the site of application without enhancing percutaneous absorption. REFERENCES (1) L. Overgaard-Olsen and G. B. Jemec, The influence of water, glycerin, paraffin oil and ethanol on skin mechanics, Acta. Derre. Venereal (Stockh), 73, 404-406 (1993). (2) E. J. Van Scott and R. J. Yu, nyperkeratinization, corneocyte cohesion and alpha-hydroxy acids, J. Am. Acad. DermatoL, 11, 867-879 (1984). (3) E. J. Van Scott and R. J. Yu, Control of keratinization with alpha-hydroxy acids and related com- pounds. I. Topical treatment of ichthyotic disorders, Arch. Dermatol., 110, 586-590 (1974). (4) M. Goldstein and R. Brucks, Evaluation of glycolic acid permeation through skin, Pharm. Res., 11, S-180 (1994). (5) C. Ackermann and G. L. Flynn, Ether-water partitioning and permeability through nude mouse skin in vitro. I. Urea, thiourea, glycerol and glucose, lnt. J. Pharm., 36, 61-66 (1987). (6) A. Rougier, D. Dupuis, C. Lotte, R. Roguet, and H. Schaefer, In vivo correlation between stratum corneum reservoir function and percutaneous absorption, J. Invest. Dermatol., 81, 275-278 (1983). (7) L. Stryer, Biochemistry, 3rd ed. (W.H. Freeman, New York, 1988), p. 392. (8) S. M. Dowton, Z. Hu, C. Ramachandran, D. F. H. Wallach, and N. Weiner, Influence of liposomal composition on topical delivery of encapsulated cyclosporine-A. I. An in-vitro study using hairless mouse skin, STP Pharma Sci., 3, 404-407 (1993). (9) S. M. Niemiec, Z. Hu, C. Ramachandran, D. F. H. Wallach, and N. Weiner, The effect of dosing volume on the disposition of cyclosporine-A in hairless mouse skin after topical application of a nonionic liposomal formulation: An in vitro diffusion study, STP Pharma Sci., 4, 145-149 (1994). (10) P. P. Sarpotdar and J.L. Zatz, Evaluation of penetration enhancement of lidocaine by nonionic surfactants through hairless mouse skin in vitro, J. Pharm. Sci., 75, 176-181 (1986). (11) K. A. Walters, M. Walker, and O. Olejnik, Non-ionic surfactant effects on hairless mouse skin permeability characteristics, J. Pharm. Pharmacol., 40, 525-529 (1987).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)