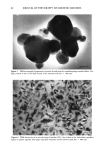
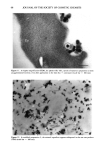
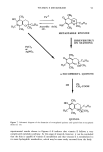
104 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS about twofold higher from Non-1 liposomes than those from Non-2 liposomes and the two solutions. Also, the total amount of glycolic acid found in the strips was roughly two times higher for Non-2 liposomes than for Non-1 liposomes. The in vitro data, therefore, supports the conclusion from in vivo studies that Non-2 liposomes allow for a greater retention of glycolic acid in the stratum corneun while retarding absorption, compared to Non-1 liposomes. Although Non-1 liposomes delivered greater amounts of glycolic acid into the living skin strata, the much higher systemic absorption of glycolic acid from Non-1 formu- lations is less desirable from a cometic formulation viewpoint. Thus, overall, the Non-2 formulation appears to be the most efficient of all the formulations tested since it allows for a greater extent of retention of glycolic acid in and on the stratum corneum as well as the living skin strata, while retarding the penetration of glycolic acid into the systemic circulation. The in vivo uptake of glycerol into the various strata of hairless mouse skin from various formulations as a function of time is shown in Table III. The accumulation of glycerol in the stratum corneum was in the order Non-1 = Non-2 30% PG solution = aqueous solution = O/W emulsion = W/O emulsion at all time points examined. The general trend for accumulation of glycerol in the living skin strata was in the order: Non-2 Non-1 O/W emulsion aqueous solution = W/O emulsion = 30% PG solution. Urinary excretion of glycerol at 8 h was in the order: Non-2 = Non-1 W/O emulsion = O/W emulsion = 30% PG solution. Since the highest total amount of glycerol in and on the stratum corneum, as estimated from the total amount of glycerol in the strippings, was obtained from Non-2 liposomes, once again Non-2 formulations appear to be the most appropriate vehicle for glycerol delivery. Table IV shows the distribution of glycerol in various strata of hairless mouse skin 16 h after topical in vitro application of select formulations. The recovery of total radioac- tivity was greater than 95% for all systems. A good linear correlation between amounts in the urinary bladder in vivo and in the receiver compartment in vitro was obtained (r 2 0.99) however, the amounts in the receiver compartment were roughly 60- to 100-fold higher. It is of interest to note that percutaneous absorption of glycerol from Non-2 liposomes is slightly higher (but not significantly) than that from Non-1 lipo- somes. The amounts of glycerol in living skin strata were similar for Non-1 and Non-2 liposomes in the in vitro experiments. Also, the amounts of glycerol in the strips were roughly threefold higher for Non-2 liposomes than for Non-1 liposomal formulations. Thus, Non-2 liposomes appear to provide better retention in the skin as well as to better facilitate deposition of glycerol into and across the skin than do Non-1 liposomes. Our studies suggest that application of nonionic liposomal formulations results in higher accumulation of glycolic acid in the living skin strata and of glycerol in the stratum corneum than that from solutions and conventional emulsions. The differences in the behavior of Non- 1 and Non-2 formulations are consistent with the mechanisms of action proposed earlier for these liposomal systems (8,9). Briefly, following topical application, both Non-1 or Non-2 liposomes undergo gradual dehydration under nonoccluded con- ditions while in contact with the skin at a temperature of 32øC. When the temperature of the dehydrating formulation exceeds the melting point of GDL (30øC), the major lipid component of Non-1 liposomes, melting of the component occurs. This results in the release of both GDL and POE-10, which are known skin penetration enhancers.
DEPOSITION OF GLYCOLIC ACID AND GLYCEROL 105 Table III Kinetics of Distribution of Glycerol (expressed as percent of applied dose -+ standard deviation) in Various Compartments of Hairless Mouse Skin After 1-Hour Topical In Vivo Application of Various Formulations (n = 3) Stratum Living Time corneum Stratum skin Urinary (h) Swabs surface corneum strata excretion Recovery Aqueous solution 0 76.6 + 2.9 13.5 -+ 1.5 5.3 + 0.4 0.60 + 0.29 ND 96.0 + 1.6 4 76.4 -+ 2.8 9.4 + 1.1 7.7 + 1.2 0.91 -+ 0.22 ND 94.4 + 0.5 8 72.7 + 2.3 11.3 -+ 1.4 5.9 -+ 1.0 0.47 -+ 0.04 ND 90.4 -+ 0.2 30% PG solution 0 69.9 -+ 9.7 22.0-+ 7.7 2.7 -+ 1.3 0.29 -+ 0.02 0.04 -+ 0.04 94.9 -+ 2.0 1 75.7 + 0.4 14.5 -+ 1.7 2.9 -+ 0.9 0.23 + 0.10 0.02 + 0.02 93.4 -+ 1.9 2 79.7 -+ 1.6 13.6 + 1.4 2.6 -+ 0.6 0.35 -+ 0.22 0.06 -+ 0.01 96.2 -+ 1.0 4 71.5 -+ 5.2 14.8 -+ 4.9 3.3 -+ 0.5 0.50 -+ 0.22 0.03 -+ 0.03 90.1 -+ 2.6 8 65.8 + 1.5 18.7 -+ 2.1 2.8 + 0.2 0.23 + 0.02 0.08 + 0.03 87.6 + 0.3 O/W emulsion 0 64.9 + 3.0 25.2 -+ 5.4 2.8 -+ 1.0 0.89 + 0.04 0.02 + 0.01 93.8 -+ 7.0 1 69.3 -+ 2.9 29.8 + 0.5 5.5 -+ 0.2 0.85 -+ 0.09 0.04 -+ 0.01 105.6 -+ 2.6 2 68.5 + 4.3 22.0-+ 4.4 4.4-+ 1.4 0.89-+ 0.15 0.03 -+ 0.01 95.8 + 2.1 4 66.8 -+ 0.7 19.1 -+ 1.4 4.3 + 0.5 0.61 + 0.06 0.17 + 0.08 91.0 + 1.3 8 63.9 -+ 2.9 11.6 + 3.3 5.9 + 0.4 0.63 -+ 0.11 0.09 -+ 0.02 82.0 -+ 6.3 W/O emulsion 0 84.8 -+ 2.5 13.0 + 1.6 1.4 + 0.2 0.11 -+ 0.02 0.03 -+ 0.02 98.9 + 1.7 1 83.6 + 2.3 13.5 -4- 0.7 2.1 --- 1.1 0.23 --- 0.09 0.02 --- 0.01 99.4 --- 1.0 2 80.7 --- 3.6 12.1 --- 2.6 3.3 --- 0.4 0.39 --- 0.07 0.01 --- 0.00 96.5 --- 1.6 4 79.5 -+ 7.7 12.6 -+ 0.5 3.8 + 0.2 0.68 + 0.09 0.04 + 0.02 96.6 -+ 7.1 8 82.7 + 4.3 8.8 + 1.1 4.2 -+ 0.0 0.39 -+ 0.01 0.21 -+ 0.06 96.3 -+ 5.4 Non- 1 liposomes 0 31.0 + 4.9 51.7 + 3.0 12.0 + 1.0 2.08 + 0.55 0.05 + 0.05 96.8 + 4.2 1 23.9 -+ 1.5 45.8 + 1.6 16.0-+ 2.3 1.44 -+ 0.36 0.08 -+ 0.06 87.3 + 2.2 2 22.9 + 9.8 40.0 -+ 4.1 20.3 -+ 4.1 1.41 -+ 0.13 0.10 -+ 0.00 84.7 -+ 0.3 4 32.3 -+ 4.4 30.0 -+ 0.9 20.1 -+ 2.4 1.07 + 0.21 0.15 + 0.06 83.7 + 2.6 8 34.5 -+ 6.0 19.6 + 6.7 19.0 -+ 4.0 1.37 -+ 0.30 0.35 -+ 0.24 74.8 + 8.6 Non-2 liposomes 0 17.2 -+ 6.1 65.3 + 6.3 14.9 + 1.8 2.13 + 0.09 0.02 + 0.01 99.6 -+ 4.5 1 17.7 -+ 0.9 59.1 -+ 3.0 14.8 -+ 1.3 2.16-+ 0.35 0.04 -+ 0.02 93.8 --- 0.6 2 18.5 + 5.1 47.0 + 3.5 19.2 -+ 3.5 2.78 -+ 0.02 0.08 -+ 0.03 87.5 -+ 1.9 4 22.0 -+ 2.8 36.9 -+ 3.2 20.9 -+ 5.7 2.19 -+ 0.75 0.31 + 0.17 82.4 + 3.9 8 21.0 -+ 4.4 30.1 -+ 4.2 19.0 + 3.3 1.98 + 0.33 0.56 + 0.37 72.7 + 2.4 Such a release of POE-10 or of GDS does not occur with Non-2 liposomes since neither GDS (m.p = 54øC) nor POE-10 (m.p = 36øC) will melt at the skin surface temper- ature of 32øC. Thus, Non-1 liposomal formulations are expected to be superior to Non-2 liposomes in facilitating transport into and across skin. It is not surprising, therefore, to find that Non-1 liposomes are better at faciltating transport of glycolic acid into and across skin. This scenario also explains why Non-2 liposomal preparations provide a better reservoir for the active and permit its sustained and steady release into the skin.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)