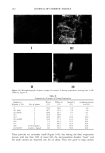
160 JOURNAL OF COSMETIC SCIENCE _ /m ' C B Figure 8. The lameliar liquid crystal is described by three zones: (A) water layer plus polar groups, (B) hydrocarbon chains, methylene groups, and (C) hydrocarbon chains, terminal methyl group, and space between them. curred, and now the continuous phase is oil with a small droplet of water. The crystals are dispersed in the continuous phase (Figure 10-III). At 0 wt% water, all water has disappeared and ot-hydroxyoctanoic acid crystals are dispersed in the oil surfactant phase (Figures 9-A and 10-IV). The composition of sample B is water (75 %), Laureth 4 (15 %), acid (5 %), and white oil (5%). The higher amount of surfactant means that the liquid crystal (LLC, Figure 5) is more prevalent and is seen to be birefringent (Fig. 11-I). Hence the entire emulsion was radiant when viewed between the crossed polarizers. Separation of the sample with low-speed centrifuge was not possible. During evaporation, the crystals of ot-hydroxy- octanoic acid appeared (Figure 11-II). They grew with continued evaporation, forming the final large crystals (Figure 11-III). Table III gives the composition of sample B during evaporation. DISCUSSION The results are of interest from several points of view. At first the phase diagram of the four components gives information about the different structures in such a system.
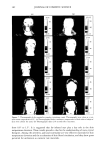
PHASE BEHAVIOR OF o•-HYDROXYOCTANOIC ACID 161 Figure 9. Photograph of sample A showing the different phases during evaporation (Table II, Figure 5). Secondly, the evaporation in combination with the phase diagram provides essential knowledge about the phases encountered during evaporation as well as the change in their composition and structure. Taken altogether, the results and their interpretation form a foundation for skin care formulations containing o•-hydroxyoctanoic acid. The results will be discussed in this order. In the phase diagrams (Figures 1-5), there are several features that have a decisive influence on the behavior of the system. One is the fact the acid dissolves significant amounts of water, oil, and surfactant simultaneously. These three compounds constitute approximately one third of the total weight, a remarkable structure with at least po- tential significance cosmetically. A second feature is the fact that there is a two-phase region in which two solutions in the L 2 region (Figure 4) are in equilibrium. The consequence of this fact is a separation into two non-aqueous phases called oil and microemulsion in the emulsion (Figure 9). This separation is important because the solid acid particles are dispersed in the microemulsion phase (Figures 9, 10). The third feature is the solubilization of the acid in the surfactant/water inverse miceliar solution (Figure 1). It is noteworthy that the acid is not soluble in the oil, or in water, or in the surfactant _per se, but is significantly so in the surfactant/water liquid. A solubility at the level of 20% is remarkable, and this microemulsion will be discussed in a subsequent publication. This solubilization is also important for the present investigation, because it affects the behavior of emulsion A during evaporation. As shown in Figures 9 and 10, the acid particles are dispersed in the microemulsion phase at the beginning of the evaporation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)