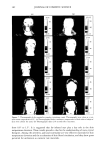
158 JOURNAL OF COSMETIC SCIENCE 75 70 65 60 'D 50 ,- 45 '• 40 35 30 25 20 0.0 ' I ' I ' I ' I 0.2 0.4 0.6 0.8 1.0 volume of water/volume of (Laureth 4+ 2-hydroxyoctanoic acid) Figure 6. Interlayer spacing versus volume ratio of water/(Laureth 4 + o•-hydroxyoctanoic acid). Alpha- hydroxyoctanoic acid/Laureth 4: ß 0/100, ß 5/95, /• 8/92, and x 10/90. increases from 40.03 • to 44.02 •. However, adding 10% changes d o from 32 • to 38.7 • with no change in the slope, as shown in Table I. The liquid crystal phase is a lameliar liquid crystal, LLC, consisting of amphiphilic bilayers separated by water layers, as shown by its optical microscope pattern (Figure 8). EMULSIONS The first sample was a multiphase emulsion, sample A, with a composition of water (70%), white oil (20%), o•-hydroxyoctanoic acid (5%), and Laureth 4 (5%). When the sample was centrifuged at low speed, three layers were observed (Figure 9). These are from bottom water (water, Figure 5), oil (white oil, Figure 5), and microemulsion (L2, Figure 5). The aqueous layer contains liquid crystals (LLC, Figure 5), and the micro- emulsion layer contains crystals of the acid (S•, Figure 5) with dissolved water, oil, and surfactant. Water evaporation showed changes in the layers and their contents (Figure 9) and in the emulsions (Figure 10). Table II gives the composition of sample A during evaporation. Figure 10 shows the emulsion to be water continuous with two kinds of droplets: oil droplets and microemulsion droplets. The dark droplets (Figure 10) are the microemulsion droplets. They are dark because of the o•-hydroxyoctanoic acid crystals inside them.
PHASE BEHAVIOR OF ot-HYDROXYOCTANOIC ACID 159 70 60 4o 30 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 volume of water/volume of [Laurcth 4+white oil] Figure 7. Interlayer spacing versus volume ratio of water/(Laureth 4 + white oil). White oil/Laureth 4: ß 0/100, ß 5/95, and x 10/90. Table I Interlayer Spacing (do) and Penetration (or) Values in LLC Laureth 4/ot-hydroxyoctanoic acid (wt ratio) Laureth 4/white oil (wt ratio) d o ot d o ot 100/0 32.5 -+ 0.7 -0.24 ñ 0.04 32.6 ñ 1.0 -0.23 ñ 0.07 95/5 27.0 ñ 3.3 -0.53 + 0.29 32.15 _+ 0.9 -0.37 ñ 0.061 92/8 28.0 _+ 1.9 -0.61 ñ 0.15 -- -- 90/10 27.9 ñ 0.9 -0.56 ñ 0.08 38.7 ñ 1.65 0.025 ñ 0.01 At 60 wt% water, the continuous phase is reduced and the droplets are crowded (Figure 10-II). The crystals of acid with dissolved water, oil, and surfactant have left the microemulsion droplets during water evaporation, unfortunately too rapidly for photo- graphs to be obtained of the process. At 14 wt% water, the number of layers is reduced to two (Figure 9, A-5). The micro- emulsion (Figure 10-III) droplets have disappeared, inversion of the emulsion has oc-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)