2 JOURNAL OF COSMETIC SCIENCE MATERIALS AND METHODS MATERIALS The preservative systems of the nine evaluated formulas were as follows: 1. Absence of preservative. 2. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.3% (v/v) methylparaben 0.2% (w/v) propylparaben 0.1% (w/v) disodium ethylenediamine-tetraacetate (EDTA) 0.2% (w/v). 3. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.0% (v/v) methylparaben 0.2% (w/v) propylparaben 0.1% (w/v) triclosan 0.2% (w/v). 4. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.3% (v/v) isopropylparaben (and) isobutylparaben (and) butylparaben (Liqua par ©) 0.3% (v/v) disodium EDTA 0.2% (w/v). 5. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.3% (v/v) dichlorobenzyl alcohol 0.15 % (v/v) disodium EDTA 0.2% (w/v). 6. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.3% (v/v) methylparaben 0.2% (w/v) propylparaben 0.1% (w/v) dichlorobenzyl alcohol 0.15% (w/v) disodium EDTA 0.2% (w/v). 7. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.3% (v/v) isopropylparaben (and) isobutylparaben (and) butylparaben (Liqua par ©) 0.3% (v/v) dichlorobenzyl alcohol 0.15% (w/v) disodium EDTA 0.2% (w/v). 8. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.0% (v/v) methylparaben 0.2% (w/v) propylparaben 0.1% (w/v) benzoic acid 0.3% (w/v) disodium EDTA 0.2% (w/v). 9. Phenoxyethanol (and) methylparaben (and) ethylparaben (and) propylparaben (and) butylparaben (Phenonip ©) 1.0% (v/v) isopropylparaben (and) isobutylparaben (and) butylparaben (Liqua par ©) 0.3% (v/v) benzoic acid 0.3% (w/v) disodium EDTA 0.2 % (w/v). METHODS Preparation and standardization of the inoculum. The following microorganisms, some from the American Type Culture Collection (ATCC) and others isolated from the product, were used in the test: Burkholderia cepacia ATCC 17759, Escherichia coli ATCC 10536, Staphylococcus aureus ATCC 6538, Shewanella putrefaciens, and Bacillus sp. These strains were used to prepare suspensions, obtained from the harvest of microbial growth of a 24-hour-culture incubated at 30ø-35øC, on the surface of tryptic soy agar (TSA, Difco Laboratories, Detroit, MI). The number of colony-forming units per milliliter (CFU/ml) of suspension was deter- mined by the pour-plate count method using the above medium and incubation con- ditions. The concentration of microorganisms in the suspensions had to be suitable to provide from 10 5 to 10 6 microorganisms per milliliter in the test preparation imme- diately after the inoculation. The standardized suspensions were used to inoculate the samples.
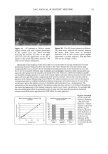
PRESERVATIVE EFFICACY TESTING 3 Challenge test through linear regression (5,6), pharmacopeial (1-3) and CTFA (4) meth- ods. Twenty-gram portions of the samples were transferred to sterile tubes and individu- ally inoculated with 0.1 ml of the standardized microbial suspensions. The inoculated tubes were stirred, and each test preparation was submitted to determi- nation of the number of viable microorganisms by the pour-plate method. The dilutions were made using a solution with 0.85% (w/v) of sodium chloride and 0.4% (w/v) of calcium chloride. The medium was tryptic soy agar (Difco Laboratories) with 0.5% (w/v) of lecithin and 0.07% of polysorbate 80. The same procedure was performed with 20 ml of the saline solutions. The number of surviving microorganisms was determined after 2, 4, 24, and 48 hours. The D-values were then calculated with a coefficient of correlation whose linear regres- sion was higher than 0.9000. A similar test was simultaneously performed whose sur- viving microorganisms, however, were determined after 7, 14, 21, and 28 days. Evaluation of the preservative systems neutralization. Lecithin 0.07% (w/v), polysorbate 80 0.5% (w/v), and calcium chloride 0.4% (w/v) were added to the diluent (0.85% solution of sodium chloride) in order to neutralize the preservative systems. The efficacy of the neutralization of the preservative systems was tested by separately inoculating the test microorganisms (1 ml of standardized suspensions containing 100 CFU/ml) into the product diluted to 10 -•. The surviving microorganisms were subsequently determined through the pour-plate method, which led to satisfactory results when the obtained CFU was similar to that of the microbial suspension. RESULTS The results obtained proved the efficacy of the neutralizing agents used. The D-values obtained for different microorganisms are shown in Table I, and the linear regressions Table I The D-Values Obtained by the Linear Regression Method Applied to One Cosmetic Formulation Without Preservative and Eight Others With Different Preservative Systems D-values (hours) Shewanella Bzzrkholderia Escherichia Staphylococczzs Bacillzzs Formula pzztrefaciens cepacia coli azzrezzs sp. I --* .... 2 4.8 18.6 -- 11.6 -- 3 17.9 -- 28.9 12.1 -- 4 -- 37.6 11.5 6.3 -- 5 11.2 -- 16.9 6.0 -- 6 5.6 -- 4.1 4.8 -- 7 5.0 58.8 14.6 8.1 -- 8 1.0 5.2 5.6 4.8 -- 9 0.9 1.3 7.5 1.7 -- * The D-value was not calculated (coefficient of correlation was lower than 0.9000).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)