DNA ANALYSIS OF HAIR FIBERS 23 for amplification of the HLA-DQA 1 genotype were (5'-3'): GGT GTA AAC TTG TAC CAG and GGT AGC AGC GGT AGA GTT G (1). Thirty cycles were carried out, consisting of denaturation at 94øC for 1 min, annealing at 54øC for 1 min, and extension at 72øC for 1 min, before a final extension step of 5 min at 72øC. Fifteen microliters of extracted hair DNA was used for amplification. To attempt to remove water-soluble melanins, some DNA samples were purified using Wizard DNA cleanup columns (Promega), according to the manufacturers instructions, before use in the PCR reaction. The resulting amplified samples were loaded on a 1.5% agarose gel, and run for 1 h at 100 V, stained with ethidium bromide, and photographed on a UV transilluminator. EFFECTS OF COLORING ON LEVELS OF HAIR SHAFT DNA Female subjects planning to have their hair permanently colored for the first time were recruited from the laboratory. Subjects had samples of hair taken before the hair treat- ment was carried out, and they returned to give a second sample after they had had their hair colored. DNA was extracted from the exact same amount of hair from each before-and-after treatment sample (around 100 mg), and the concentrations of DNA were determined by using the Pico-green © assay and related back to the exact amount of starting material. DNA LEVELS IN ROOT-END VERSUS TIP-END OF HAIR SHAFTS Root-end and tip-end hair was separated from 1-g Spanish hair switches (International Hair Importers). DNA was extracted from the same amount of hair for root and tip-end hair (around 100 mg), and the concentrations of DNA were determined by using the Pico-green © assay and related back to the exact amount of starting material. EFFECTS OF SHAMPOO WASHING ON DNA LEVELS OF HAIR SHAFTS Four samples of 50 mg of finely cut root-end switch hair were washed once for 30 sec in 1 ml of a 1:10 dilution of 12:2 base shampoo (12% sodium lauryl ethyl sulfate, 2% tegobetaine), and the wash solution was removed for purification and quantitation of DNA. Four samples of 50 mg of finely cut root-end switch hair were washed five, ten, or 20 times for 30 sec each time in 1 ml of a 1:10 dilution of 12:2 base, with 30-sec rinses in distilled water between each wash. The washes were pooled, and a 1 ml solution was removed for purification and quantitation of DNA. Four samples of 100 mg of finely cut switch hair was soaked in 2 ml of a 1:10 dilution of 12:2 shampoo base for 2 h. The wash solution was removed for purification and quantitation of DNA. The DNA concentrations were determined using the Pico-green © reagent, and the total amount of DNA lost during each washing procedure was calculated.
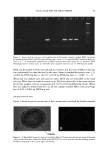
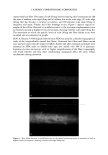
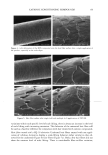
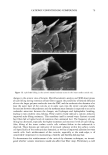
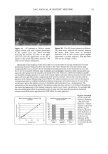
24 JOURNAL OF COSMETIC SCIENCE STATISTICAL ANALYSIS Differences in levels of DNA were determined by carrying our paired t-tests using the JMP statistical package (SAS Institute Inc.) for the before-and-after coloring and when comparing root and tip levels of DNA from the same switch. LOCALIZATION OF DNA IN HAIR SHAFTS Approximately 20 hairs, 1.5 cm in length, were randomly cut from hair switches. These cut lengths were stained for 2 h at room temperature in 10 pM of TOTO-3 © (double stranded DNA-specific stain from Molecular Probes). After washing, the stained hair samples were immediately embedded in Agar 100 epoxy resin, and polymerized overnight at 60øC. Thin 4-1am cross sections were then cut using a Histo diamond knife. These sections were mounted in water on glass microscope slides and gently heated in order to optimally fiatten the mounted sections. For microscope examination, the dry fiat resin-embedded hair sections were mounted in glycerol, cover slipped, and finally sealed with nail varnish. Hair sections were also cut first and the staining carried out afterwards in the same way. All hair sections were examined using a computerized Olympus Provis AX microscope equipped with brightfield, differential interference contrast, and epi-illumination fluo- rescence observation modes. In order to observe cuticle detail, an auxiliary x2 zoom lens and a x5 projection eyepiece were used. The fluorescence filter assembly suitable for TOTO-3 © was obtained from Omega Optical. Unstained hair sections were almost completely dark, indicating negligible hair autofluorescence backgrounds. Thus all significant fluorescence intensities measured in this work relate solely to TOTO-3 © fluorescence emission and were background-free. RESULTS IDENTIFICATION OF DNA FROM HAIR SHAFTS The Pico-green © assay of the DNA extracted from hair confirmed the presence of double-stranded DNA. From a single hair, approximately 0.4 ng of DNA was extracted. From 0.2 g of hair, the amount of DNA extracted was between 12 ng and 50 ng. Variation in levels of DNA was seen between different hairs from the same head, as well as between individuals, but DNA was always present. PCR products were clearly visible for three of the samples of DNA extracted from hair for the HLA-DQA1 locus, giving a product of 241 bp. Very low concentrations of DNA and samples that had not been subjected to a cleanup column did not amplify. A gel showing the successful amplification for the HLA-DQA1 gene is shown in Figure 1. EFFECTS OF COLORING AND WASHING ON LEVELS OF SHAFT DNA Levels of DNA were significantly reduced after permanent colorant treatment of hair. Mean (+standard deviation): before treatment 0.94 + 1.53 ng DNA/mg hair vs after treatment 0.74 + 1.08 ng DNA/mg hair, p 0.05, n = 18.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)