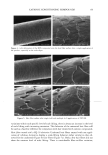
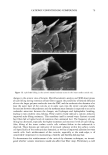
j. Cosmet. sci., 54, 21-27 (January/February 2003) Analysis of DNA in hair fibers DANIELLA M. HEYWOOD, RICHARD SKINNER, and PAUL A. CORNWELL, Hair Group, Unilever Research, Port Sunlight, Quarry Road East, Bebington, Wirral, CH63 3JW, United Kingdom. Accepted for publication September 6, 2002. Synopsis The extraction and identification of deoxyribonucleic acid (DNA) from human hair shafts is described, along with the effects of hair treatments on levels of DNA and suggestions of DNA location within the shaft. DNA was present at low levels in the hair shaft, and was identified using polymerase chain reaction amplification of the human leukocyte anrigen (HLA)-DQA1 locus. The use of cleanup columns aided the success of PCR amplification. DNA appears to reside in the cuticle portion of the hair shaft. Levels of DNA were found to be higher at the root-end compared to the tip-end of hair and were also found to be lower after permanent colorant treatment. DNA was found to be lost with surfactant washing, with increased loss occurring with prolonged or an increasing number of washes. These results suggest that small amounts of residual DNA remain after differentiation and add to our knowledge of the constituents of hair. INTRODUCTION The analysis of deoxyribonucleic acid (DNA) from intact roots of single hair fibers is now routinely used in the area of forensic science. While the detection of mitochondrial DNA from hair shafts is quite successful (1), the detection of genomic DNA from hair shafts without the presence of an intact root is far less reliable, but has been carried out by a few research groups (2-5). Contrary to previous thoughts, results demonstrating the presence of high-molecular-mass genomic DNA in hair shafts (5) suggest that not all genomic DNA degenerates during the keratinization process. The level of DNA in hair shafts has been shown to be residual compared to the average 0.5 l•g found in intact hair follicles (6). These low levels, combined with the fact that water-soluble melanins, responsible for the pigment of hair, inhibit DNA polymerase enzymes, make DNA analysis from hair shafts difficult (7). These water-soluble melanins are produced spontaneously within the hair fiber, but levels are provoked by hydrogen peroxide, a component of permanent colorants and bleaches (7). Address all correspondence to D. M. Heywood. 21
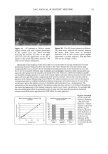
22 JOURNAL OF COSMETIC SCIENCE In the present study, procedures were established to extract DNA from hair fibers, to confirm that extracted DNA was of human origin using the Pico-green © reagent (PCR), and to quantify DNA in extracts. Sodium dodecyl sulfate (SDS) washes have been shown to reduce the level of DNA in hair (5). Experiments were therefore carried out to investigate the effects of common basic shampoo on the level of DNA in hair. DNA has been isolated specifically from hair cuticle (2), suggesting that this may be the location of DNA within the hair shaft. Experiments were therefore also carried out to investigate the location of DNA within the shaft using DNA-specific fluorescent stains and fluorescence microscopy. METHODS EXTRACTION OF DNA FROM HAIR SHAFTS Freshly clipped hair was washed in ethanol for 5 min and rinsed in distilled water before being cut as finely as possible with hair scissors, and digested overnight at 56øC in 0.01 M Tris-HCl (pH 8), 0.005 M EDTA, 0.1 M NaC1, 0.039 M DTT, 2% SDS, and 250 lag of proteinase K (Gibco) per ml of extraction buffer. The digested hair solution was then extracted twice with two volumes of phenol satu- rated by Tris-EDTA (TE) buffer (Sigma), and once with two volumes of chloroform. The solutions were centrifuged between each extraction. The resulting DNA in the final aqueous phase was then precipitated using two and a half volumes of ice-cold absolute ethanol. The DNA was pelleted by centrifugation, and the resulting pellet washed in 70% ethanol and centrifuged for a further 10 min. The DNA pellet was air-dried for 20 min before being resuspended in 100 lal of TE buffer for the Pico-green © assay (Mo- lecular Probes), or 15 lal for PCR. The method was based on that previously described by Uchihi et •L (2). Gloves were used at all times to prevent DNA from the hands contaminating samples. IDENTIFICATION OF EXTRACTED DNA FROM HAIR SHAFTS The concentration of DNA was determined using the Pico-green © reagent, which is a fluorescent nucleic acid stain for specifically quantitating double-stranded DNA. Vari- ous amounts of clipped hair were used from one hair (approximately 1 mg) up to 200 mg of hair. The unknown concentrations of DNA were determined from extracted hair samples by using a spectrofluorimeter (Spex Fluoromax) according to the manufacturers instruc- tions, employing human genomic DNA for calibrations (Promega), and were calculated in relation to the amount of starting material (ng of DNA per mg of hair). PCR AMPLIFICATION OF THE HLA-DQA1 GENE PCR reactions consisted of 1 x PCR buffer (10 mM of Tris-HC1 [pH 8.3], 500 mM of KCI), 1.5 mM of MgCl2, 200 laM of each dNTP (dCTP, dGTP, dATP, dTTP), 50 pmol of each primer, and 1.25 units of Amplitaq in a volume of 25 lal. The primers designed
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)