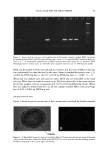
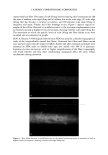
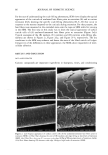
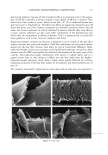
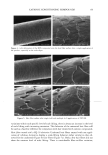
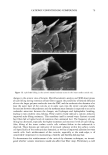
DNA ANALYSIS OF HAIR FIBERS 27 cell nucleus (8) and that this would be a logical portion of the fiber to contain nucleic acid material. Fluorescence microscopy demonstrated for the first time that DNA does appear to be present in the cuticle of the hair fiber, although the exact location within the cuticle could not be determined at this level of magnification. Histochemical studies have shown nuclear remnants to be present in both the cuticle and cortex (8). Microscope and epi-illumination observations carried out in-house show disk-like remains of the cell nucleus in the endocuticle, pushed to one side as the A-layer, exocuticle, and inner layer were formed in the hair follicle. The endocuticle is the least organized of all the hair structures, and so more likely to have retained original cell organelles. The cortex is much more crystalline, and so any nuclear remnants are likely to be eliminated as the dense microfibrillar structures become set. The cuticle also keratinizes quite early, and so there may be less time for endonucleases to destroy DNA. These findings are further evidence that DNA is present in mature hair fibers and appears to reside in the cuticle. DNA is also affected by cosmetic procedures, including shampoo washing and permanent coloration. These findings add to our knowledge of the biological composition of hair and how it is affected by potentially damaging procedures. ACKNOWLEDGMENTS We thank Mr. M. Ashton for carrying out the fluorescence microscopy and Ms. N. Noel for technical assistance with the extraction and measurement of DNA. REFERENCES (1) M. R. Wilson, D. Polanskey, J. Butler, J. A. DiZinno, J. Replogle, and B. Budowle, Extraction, PCR amplification and sequencing of mitochondrial DNA from human hair shafts, BioTechniques, 18, 662-669 (1995). (2) R. Uchihi, K. Tamaki, T. Kojima, T. Yamamoto, and Y. Katsumata, Deoxyribonucleic acid (DNA) typing of human leukocyte antigen (HLA)-DQA 1 from single hairs in Japanese, J. Forensic Sci., 37, 853-859 (1992). (3) J. Kalbe, R. Kuropka, L. S. Meyer-Stork, S. L. Sauter, P. Loss, K. Henco, D. Riesner, H. Hocker, and H. Berndt, Isolation and characterization of high-molecular-mass DNA from hair shafts, Biol. Chem., 369, 413-416 (1988). (4) R. Higuchi, C. H. von Beroldingen, G. F. Sensabaugh, and H. A. Erlich, DNA typing from single hairs, Nature, 332, 543-545 (1988). (5) A. Schreiber, E. Amtmann, V. Stoerch, and G. Sauer, The extraction of high-molecular-mass DNA from hair shafts, FEBS Lett., 230, 209-211 (1988). (6) M. W. A. C. Hukkelhoven, E. Vromans, A.M. G. Markslag, A. J. M. Vermorken, A simple fluoff- metric microassay for DNA in hair follicles or fractions of hair follicles, Anticancer Res., 1, 341-344 (1981). (7) T. Yoshii, T. K. Akiyama, K. Tamura, I. Ishiyama, PCR inhibitor: Water-soluble melanin, which inhibits DNA polymerases and DNases, Adv. Forensic Haemogenetics, 5, 393-396 (1994). (8) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 3rd ed. (Springer-Verlag, New York, 1994). (9) K. Kizawa, T. Takizawa, T. Inoue, I. Sasaki, M. Yamaguchi, and H. Uchiwa, New model for hair damage: The key role of S100A3 expressed in cuticles, Proceedings of the 20th IFSCC Congress, P014, 1-8 (1999).
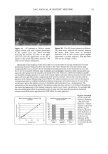
j. Cosmet. Sci., 54, 29-46 (January/February 2003) Challenging the surfactant monomer skin penetration model' Penetration of sodium dodecyl sulfate micelies into the epidermis PETER N. MOORE, SUDHAKAR PUVVADA, and DANIEL BLANKSCHTEIN, Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 (P.N.M., D.B.), and Unilever Home and Personal Care NA, Trumbull, CT 06611 (S.P.). Accepted for publication November 15, 2002. Synopsis The penetration of sodium dodecyl sulfate (SDS) into the epidermis was measured using •4C-radiolabeled SDS. It was found that, at surfactant concentrations that exceed the critical micelle concentration (CMC) of SDS, the concentration of SDS measured in the epidermis increased as the total SDS concentration in the solution contacting the skin increased, thus demonstrating that miceIlar SDS contributes to the penetration of SDS into the epidermis. The observed SDS dose-dependent response contradicts the widely accepted view that only surfactant monomers penetrate into the skin, while surfactant in micellar form does not contribute to surfactant penetration into the skin. Nevertheless, this finding is consistent with previously unexplained observations of a dose-dependent damage to the skin induced by SDS at concentrations above the CMC. When poly(ethylene oxide) (PEO) was mixed with SDS, SDS micelles bound to PEO did not contribute to the concentration of SDS in the epidermis, while SDS in free SDS micelles did. Dynamic light-scattering measurements revealed an average hydrodynamic radius of 20 ]k for the SDS micelies, and a larger radius of 25 ]k for the PEO-bound SDS micelies. A comparison with typical aqueous pore radii in the stratum corneum measured in the literature (10-28 ]k) suggests that the SDS micelles may be able to penetrate into the skin, while the PEO-bound SDS micelles may be sterically hindered from penetrating into the skin. INTRODUCTION The investigation of surfactant-induced skin irritation has been extensive, including (i) which surfactants are most irritating (1-8), (ii) how mixing surfactants can reduce skin irritation (9-11), and (iii) how surfactants can lead to changes in the permeability of the skin (12-18). All the proposed mechanisms for surfactant-induced skin irritation involve the penetration of surfactants into the skin, where they can (i) denature proteins (2,4,19- 21), (ii) remove lipids from the stratum corneum (SC) (22-25), or (iii) disrupt the Address all correspondence to Daniel Blankschtein. 29
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)