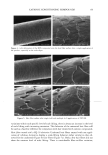
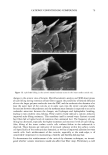
30 JOURNAL OF COSMETIC SCIENCE integrity of the lipid bilayers in the SC and the viable epidermis (12,16-18,26,27). The widely accepted view regarding surfactant-induced skin irritation is that, at surfactant concentrations that exceed the critical micelie concentration (CMC), where surfactant micelies first form, only surfactant monomers can penetrate into the skin, either because surfactant micelies are not surface-active, or because they are too large to penetrate into the SC (2,5,7,9,10,15,28,29). This view is based primarily on experimental observations using mixtures of surfactants, where surfactant-induced skin irritation was correlated with the CMC of the surfactant mixtures examined (5,9-11). This correlation is viewed as evidence that only the surfactant monomers are responsible for skin irritation, because the CMC is approximately equal to the surfactant monomer concentration. We call this widely accepted view the surfactant monomer skin penetration model and will refer to it hereafter as the monomer penetration model If surfactant-induced skin irritation is controlled solely by the monomeric surfactant, then it would naturally follow that there should be no effect of increasing the total surfactant concentration beyond the CMC, since the surfactant monomer concentration should remain approximately constant in that case. Instead, it has been observed ex- perimentally that as the total surfactant concentration is increased beyond the CMC, the surfactant-induced damage to the skin increases as well (1-3,7,10,13,15). For example, Agner and Serup found that the severity of the transepidermal water loss (TEWL) induced by the anionic surfactant SDS increased as the SDS concentration increased beyond the CMC of SDS (8.7 mM) (1). In other studies, it was found that as the SDS concentration increased beyond the CMC, the amount of SDS that penetrates into the stratum corneum also increased (2,28,29). Yet another illustration of the effect of increasing the total surfactant concentration above the CMC is provided by Rhein et aL (10). Specifically, these authors demonstrated that mixing SDS with a second, milder anionic surfactant, alkyl 7-ethoxy sulfate (AEOS- 7), led to a reduction in the irritation potential of SDS that correlated with a reduction in the CMC of the surfactant mixture, thus providing evidence for the monomer pen- etration model. However, the authors also noted that when the total surfactant concen- tration was increased, the irritation also increased, a finding that is not consistent with the monomer penetration model and that suggests a more complex mechanism of surfactant-induced skin irritation. Various explanations have been put forward to rationalize the observed dose-dependent effect of surfactants on the skin at surfactant concentrations above the CMC, including (i) that the monomer activity increases above the CMC (15) and (ii) that the micelies in the contacting solution solubilize lipids present in the skin (22,28). However, regarding explanation (i), various measurements, including surface tension and solution conduc- tivity, clearly indicate that the surfactant monomer activity remains fairly constant, or even decreases, as the surfactant concentration increases beyond the CMC (30,31). With regard to explanation (ii), there is evidence that micelies are able to remove some lipids from the skin, but the observed changes in the skin lipid concentration and composition are rather small and difficult to interpret (22-25). According to explanation (ii), an increase in the number of micelies above the CMC should lead to an increase in the lipid solubilization capacity of the contacting solution, thus explaining the increased damage to the skin, but not explaining the observed increased penetration of SDS into the stratum corneum observed by Faucher and Goddard (29), and by Ananthapadmanabhan et aL (28). In her comprehensive review of surfactant interactions with the skin, Rhein
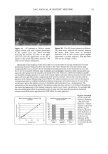
EPIDERMAL PENETRATION OF SDS MICELLES 31 stated that the observed dose dependence of surfactant-induced skin irritation beyond the CMC cannot be explained solely by the contribution of the monomeric surfactant, and speculated that the dose-dependence may result from the penetration of submicellar aggregates into the skin (32). However, in this paper, we present evidence that the mice//ar surfactant contributes to surfactant penetration into the skin, and investigate the factors that control the penetration of both miceliar and monomeric surfactant into the epidermis. With this in mind, we have measured the amount of epidermis, consisting of the SC and the viable epidermis, aqueous SDS solutions of increasing SDS concentration. centration of SDS in the epidermis is directly related to micelies in the contacting solution. We have also found SDS that penetrates into the after five hours of exposure to We have found that the con- the concentration of free SDS that, in the presence of poly- (ethylene oxide), the SDS that complexes with PEO in the form of PEO-bound SDS micelies cannot penetrate into the epidermis, while the free, or unbound, SDS micelies can. Using dynamic light scattering (DLS), we will show that a plausible explanation of our findings is that the free SDS micelies can penetrate into aqueous pores present in the SC, while the PEO-bound SDS micelies are sterically hindered from penetrating into the SC. EXPERIMENTAL MATERIALS Sodium dodecyl sulfate (SDS) and poly(ethylene oxide) (PEO) (molecular weight of 8000 g/mol) were purchased from Sigma Chemical Company (St. Louis, MO) and used as received. Water was produced using a Millipore Academic water filter. •4C-radiolabeled SDS was purchased from American Radiolabeled Chemicals (St. Louis, MO) and used as received. Phosphate-buffered saline (PBS) was prepared using PBS tablets from Sigma and Millipore-filtered water. PREPARATION OF SKIN SAMPLES Female Yorkshire pigs (40-45 kg) were purchased from local farms, and the skin (back) was harvested within one hour after sacrificing the animal. The subcutaneous fat was trimmed off using a razor blade, and the full-thickness pig skin was cut into small pieces and stored in a -80øC freezer for up to two months. The surfactant penetration experi- ments were performed using full-thickness pig skin. EXPERIMENTAL PROTOCOL The pig skin was mounted in a vertical Franz diffusion cell (Permegear Inc., Riegelsville, PA), with the SC side facing the donor compartment. Phosphate-buffered saline (PBS phosphate concentration of 0.01 M NaCI concentration of 0.137 M Sigma Chemical Company, St. Louis, MO) was added to the donor and the receiver compartments of the diffusion cell, and the skin was allowed to hydrate for one hour. The PBS in the donor compartment was removed, and 1.5 ml of surfactant solution was added to the donor
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)