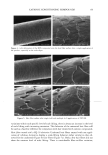
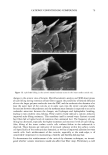
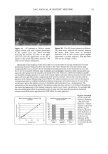
42 JOURNAL OF COSMETIC SCIENCE SDS submicellar aggregates contribute to SDS skin penetration only in the absence of PEO. Due to the hydrophilic nature of micelies, micelles would be expected to enter the SC through aqueous pathways (pores) rather than through non-polar pathways (53). Aque- ous pathways in the SC are believed to be located in the intercellular region, in particular in the lacunae and other aqueous regions surrounded by polar lipids (53-56). The characteristic radius of the aqueous pores in the skin has been determined using hin- dered-transport theories (12,42-44). Typical values for the aqueous pore radii of human skin range from 10 to 25 fit (12,43,44). For the Yorkshire pig skin used in this paper, the characteristic aqueous pore radius was determined to be about 28 fit (42). Peck eta/. (12) observed that the average pore size of the SC did not change significantly when the epidermis was exposed to SDS solutions for 18 hours. Instead, they concluded that the increased permeability of the skin resulted from an increase in the effective porosity/ tortuosity of the SC. This observation is relevant to our findings because it indicates that exposing the epidermis to an SDS solution will not change the aqueous pore sizes available for penetration into the skin. Nevertheless, we believe that additional research should be conducted to better understand the effect of surfactant penetration into the skin on the aqueous pathways of the SC. In Figure 6 we determined that the average radius of the free SDS micelies is 20 fit. These micelies are small enough that they could conceivably penetrate into the aqueous pores of the skin. However, according to our light-scattering studies, the PEO-bound SDS micelies have an average radius of 25 fit, and would be sterically hindered from passing into most aqueous pores in the skin since they are of about the same size as these pores. Consequently, according to our hypothesis, the ability of the micelies to affect the amount of surfactant penetrating into the skin is determined by the size of the micelie relative to the aqueous pore size. In this respect, it should also be recognized that both the micelies and the aqueous pores exhibit a range of sizes (31,57). It is expected that the micelies will only be able to penetrate into the skin when there is an overlap in these size ranges. Accordingly, although some aqueous pores may be large enough to allow the penetration of PEO-bound SDS micelies, such events would be relatively rare. In general, one should keep in mind that the aqueous pores in the skin are negatively charged (12,41). As a result, in addition to the micelie steric hindrance considerations put forward in this paper, there may be cases where electrostatic interactions between charged micelies and the charged aqueous pores need to be considered to determine the ability of charged micelies to penetrate into the aqueous pores. However, such electro- static effects are not expected to play a role in the studies reported in this paper because the free SDS micelies and the PEO-bound SDS micelies should have the same net negative charge, since PEO is a neutral molecule and its complexation with SDS does not screen the negatively charged sulfate groups of SDS. In addition, PEO is not expected to modify the negative charge of the aqueous pores. Consequently, the observed exclu- sion of the PEO-bound SDS micelies from the aqueous pores, coupled with the observed inclusion of the free SDS micelies into the aqueous pores, should result entirely from the proposed micelie steric hindrance mechanism embodied in penetration model (iii). Future work aimed at studying the effect of electrostatics on permeant penetration into the epidermis should consider the skin penetration of fixed-size charged species at different ionic strengths.
EPIDERMAL PENETRATION OF SDS MICELLES 43 CONCLUSIONS According ro a widely accepted view, suroeacranr micelies cannot penetrate into the skin, due ro size limitations or other oeacrors, and as a result, suroeacranr-induced skin irritation should be determined solely by the concentration ooe suroeacranr toohomers (2,5,7,9,10,15). •Vde have shown rhar this is nor the case for SDS. Instead, for a fixed amount ooe rime (five hours), we found rhar the amount ooe SDS rhar penetrates into the skin is directly related ro the concentration ooe SDS micelies present in the solution contacting the skin. •Vdhen PEO is added ro the SDS solution, SDS in micelies rhar are bound ro PEO does nor penetrate into the skin, while toohomeric SDS and SDS in free micelies do. A regression analysis of our experimental data indicated rhar the toohomeric SDS penetrates into the skin between two and three rimes oeasrer than the SDS in free micelies, while SDS bound ro PEO does nor penetrate into the skin significantly. However, ar the relatively high suroeacranr concentrations typically encountered in prac- tical applications, 5-10 wr% (where 1 wr% SDS corresponds ro 35 raM), the miceliar contribution will overwhelm rhar ooe the toohomers. A new model of suroeacranr penetration into the skin was required ro explain these results, since the current toohomer penetration model was unable ro do so. The model had ro be able ro explain both why SDS from free micelies is able ro penetrate into the skin, and why SDS from PEO-bound SDS micelies is unable to. •Vde proposed a new model ro explain these results, in which the free SDS micelies are able ro penetrate into the skin, while the PEO-bound SDS micelies are nor. SDS micelies are very hydrophilic, such rhar any penetration into the stratum comeurn will require a hydrophilic pathway. Such a pathway exists in the aqueous pores rhar are present in the stratum comeurn (53-56). In our new suroeacranr skin penetration model, the free SDS micelies can access the aqueous pores while the PEO-bound SDS micelies cannot. •Vde propose rhar the ability ro access the aqueous pores is determined by the size ooe the SDS micelie, or ooe the PEO-bound SDS micelie, relative ro the skin aqueous pore size. Several researchers have measured the average aqueous pore radius ooe the skin, and found ir ro be between l0 and 28 • (12,42-44). •Vde have measured the average radius ooe the SDS micelies, both in the presence and in the absence ooe PEO, and found rhar the free SDS micelies have a radius ooe about 20 •, while the PEO-bound SDS micelies have a radius ooe about 25 •. Therefore, the oeree SDS micelies are small enough ro access the aqueous pores, while the PEO-bound SDS micelies are srericMly hindered from pen- erraring into the aqueous pores of the skin. Although our results contradict the widely accepted view rhar suroeacranr in miceliar form does nor contribute ro suroeacranr penetration into the skin, there is ample evidence in the literature rhar the damage ro the skin is related ro the concentration ooe miceliar SDS in the contacting solution (1-3,7,10,13,15). •Vde have shown rhar this damage can be explained by the increased penetration ooe SDS into the skin, and we have also shown rhar this penetration can be reduced by changing the solution characteristics ooe the SDS micelies through the addition ooe PEO. Our findings and proposed new model ooe suroeacranr penetration into the skin should also be useful from a practical viewpoint, as they provide a new operarionM strategy for reducing the penetration ooe a known irritant (SDS) into the skin, which will hopefully reduce the damage caused by SDS through a reduction in its dose in the skin. In this new strategy, one must consider the possible contribution of the suroeacranr micelies, in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)