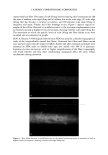
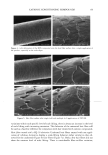
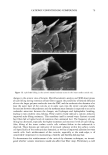
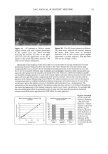
40 JOURNAL OF COSMETIC SCIENCE NEW PROPOSED MODELS OF SURFACTANT PENETRATION INTO THE EPIDERMIS We have demonstrated that the amount of SDS that penetrates into the epidermis is determined by both the concentrations of SDS monomers and of free SDS micelies in the contacting solution, and that the concentration of SDS micelies bound to PEO has no effect on SDS penetration into the skin. Because, as we have shown, the monomer penetration model cannot explain the experimental observations in Figures 1, 2 and 5, we next attempted to identify a surfactant skin penetration model that would be con- sistent with our experimental observations. We believe that the key to the new surfac- tant skin penetration model lies in the different behaviors observed for SDS in free micelles and in SDS micelies bound to PEO, since the former contribute to surfactant penetration into the epidermis while the latter do not contribute, as shown by the regression analysis. Below, we discuss three possible surfactant skin penetration models to explain our experimental observations: (i) micelie kinetics affect the rate of surfactant monomer replenishment, (ii) micelies disintegrate when they approach the skin, and (iii) micelies can penetrate into the skin, but there is a size limitation. Penetration model (i) utilizes the kinetics of micelie dissolution (48,49) to explain the observed increase in SDS penetration into the epidermis with increasing SDS concen- tration in the contacting solution, and is an extension of the original monomer pen- etration model. In this model, as the SDS monomers in the contacting solution penetrate into the SC, they must be replenished, either by the breakup of SDS micelies into monomers or by the diffusion of other SDS monomers present in the contacting solution farther away from the SC. As the SDS micelie concentration in the contacting solution increases, the argument goes, the rate of monomer formation through micelie dissolution should increase, and hence the concentration of SDS monomers adjacent to the SC should be higher in the presence of more SDS micelies. As appealing as this model appears initially, it fails in several respects. First, it is well known that as the SDS concentration in pure water increases from 50 to 200 mM, the rate of monomer formation through micelie dissolution actually decreases, with the SDS micelies at 200 mM being the most stable (50). Therefore, as the concentration of SDS in the contacting solution is increased, the rate of SDS monomer formation should decrease. According to this kinetic model, the SDS penetration into the epidermis should actually decrease, because the concen- tration of SDS monomers adjacent to the SC will be lower as the concentration of SDS in the contacting solution increases. A typical time constant for the breakup of SDS micelles to replenish the decreased SDS monomer supply, as SDS molecules penetrate into the skin, is between 0.01 s and 1 s (50). The characteristic time constant for diffusion across a membrane is described by L2/D, where L is the membrane thickness and D is the rate of diffusion through the membrane. The SC is about 15-pm thick, and the diffusion of surfactants across the SC is at least 1000 times slower than in bulk water (2), or approximately 10-•3 m2/s. This yields a characteristic diffusion time constant of about 2000 s, several orders of mag- nitude slower than the replenishment rate of the SDS monomers as SDS molecules penetrate into the SC. This simple timescale analysis suggests that the rate-determining step of diffusion into the skin is governed by the diffusion through the stratum corneum, and not by the micelie kinetics. Another problem with the micelie-kinetic model is that the addition of PEO to a SDS solution has been shown to increase the rate of micelie dissolution dramatically (51,52).
EPIDERMAL PENETRATION OF SDS MICELLES 41 Therefore, if micelie kinetics controlled the penetration of SDS into the epidermis in the presence of PEO, the penetration of SDS into the epidermis should increase as more SDS is added. Accordingly, one should observe an increase in the SDS concentration in the epidermis in Figure 2 as one goes from 50 mM SDS to 100 mM SDS in the presence of PEO. Instead, PEO prevents the PEO-bound SDS micelies from penetrating into the skin, as determined by the regression analysis presented above, thus making penetration model (i) inconsistent with the experimental observations. Penetration model (ii) assumes that the micelies are not stable when they approach the skin, and instead break apart when they impinge on the skin. In order for this mecha- nism to operate, the PEO-bound SDS micelies must somehow be prevented from con- tributing to this mechanism, while the free SDS micelles must be able to do so, evenwhen the two micelle types are presen simultanously in the conactin solution In refuting mechanism (i), we argued that the kinetics of SDS micelie dissolution are accelerated in the presence of PEO (51,52). This decreased micelle stability demonstrates that PEO-bound SDS micelies are less stable than free SDS micelies, and hence should be more likely to disintegrate as they impinge on the skin surface, which is contrary to the observed lack of contribution of the PEO-bound SDS micelies to SDS skin penetration. Accordingly, penetration model (ii) does not appear to adequately explain the observed dose- dependent penetration of SDS into the epidermis. Penetration model (iii) assumes that, contrary to the monomer penetration model, SDS micelies are actually able to penetrate into the SC. Although it is not expected that a SDS micelie would pass through the SC without breaking up due to its self-assembling nature, if the SDS micelies could penetrate even partially into the SC, then the concen- tration of SDS in the epidermis could be increased dramatically. If the free SDS micelles could penetrate into the SC while the PEO-bound SDS micelles could not, then the concentration of SDS in the epidermis would be related to the concentration of SDS in the free micelies and not to the concentration of SDS bound to PEO, as we observed experimentally in Figures 1, 2, and 5. Therefore, our hypothesis that some micelies can penetrate into the SC, while others cannot, appears to be consistent with our experi- mental observations. The concept proposed in this paper that micelies can penetrate into the skin differs from the earlier speculation that the submicellar aggregates are responsible for the observed dose dependence (32). In the case of SDS, the concentration of submicellar aggregates (aggregates composed of less than about 30 SDS molecules that do not constitute a complete micelie) does not increase significantly with total SDS concentration beyond the CMC (32). However, according to the results of our regression analysis presented above, the contribution of the non-monomeric fraction of SDS to SDS penetration into the skin (represented by b in Eq. 3) is between a third and a quarter of the monomeric contribution (represented by a in Eq. 3). The low concentration of submicellar aggre- gates, predicted by kinetic theories of micellization (48), is not sufficient to explain the large contribution of the free SDS micelies to SDS penetration into the skin. Indeed, the submicellar aggregates represent a very small fraction of the total non-monomeric SDS population, with the free SDS micelies accounting for the large majority of this popu- lation. If only the submicellar aggregates were responsible for the dose-dependent SDS penetration into the skin, then the rate of submicellar SDS penetration into the skin would have to exceed the SDS monomeric rate by several orders of magnitude to account for the observed SDS dose dependence. Finally, one would also need to explain why the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)