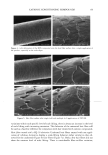
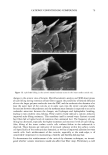
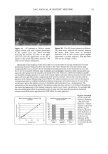
46 JOURNAL OF COSMETIC SCIENCE (35) (36) (37) (38) (39) (40) (41) W. Brown, J. Fundin, and M. G. Miguel, Poly(ethylene oxide)-sodium dodecyl sulfate interactions studied using static and dynamic light scattering, Macromo/ecMes, 25, 7192-7198 (1992). P.J. Missel, N. A. Mazer, G. B. Benedek, and M. C. Carey, Influence of chain length of the sphere- to-rod transition in alkyl sulfate micelles,J. Phys. Chem., 87, 1264-1277 (1983). A. Rohde and E. Sackman, Quasieleatic light-scattering studies of miceliar sodium dodecyl sulfate solutions at the low concentration limit, J. Colloid Inte•ace Sci., 70, 494-505 (1979). P. L. Dubin, J. H. Grubher, J. Xia, and H. Zhang, The effect of cations on the interaction between dodecylsulfate micelies and poly(ethyleneoxide),J. Colloid Interface Sci., 148, 35-41 (1992). Y. J. Nikas and D. Blankschtein, Complexation of nonionic polymers and surfactants in dilute aqueous solutions, Langmuir, 10, 3512-3528 (1994). W. M. Deen, Hindered transport of large molecules in liquid-filled pores, AIChEJ., 11, 1306-1314 (1987). P. M. Lai and M. S. Roberts, An analysis of solute structure-human epidermal transport relationships in epidermal iontophoresis using the ionic mobility: Pore model, J. Controlled Re/ease, 58, 323-333 (1998). (42) H. Tang, S. Mitragotri, D. Blankschtein, and R. Langer, Theoretical description of transdermal transport of hydrophilic permeants: Application to low-frequency sonophoresis, J. Pharm. Sci., 90, 545-568 (2001). (43) K. D. Peck, V. Srinivasan, S. K. Li, W. I. Higuchi, and A.-H. Ghanem, Quantitative description of the effect of molecular size upon electroosmotic flux enhancement during iontophoresis for a synthetic membrane and human epidermal membrane, J. Pharm. Sci., 85,781-788 (1996). (44) S. K. Li, W. Suh, H. H. Parikh, A.-H. Ghanem, S. C. Mehta, K. D. Peck, and W. I. Higuchi, Lag time data for characterizing the pore pathway of intact and chemically pretreated human epidermal mem- brane, Int. J. Pharm., 170, 93-108 (1998). (45) S. Patil, P. Singh, K. Sarasour, and H. Maibach, Quantification of sodium lauryl sulfate penetration into the skin and underlying tissues after topical application--Pharmacological and toxicological implications,J. Pharm. Sci., 84, 1240-1244 (1995). (46) J. Xia, p. L. Dubin, and Y. Kim, Complex formation between poly(oxyethylene) and sodium dodecyl sulfate micelies: Light scattering, electrophoresis, and dialysis equilibrium studies,J. Phys. Chem., 96, 6805-6811 (1992). (47) J. R. Welty, C. E. Wicks, and R. E. Wilson, Fundamentals of Momentum, Heat, and Mass Transfir, 3rd ed. (John Wiley & Sons, New York, 1984). (48) E. A. G. Aniansson, S. N. Wall, M. Almgren, H. Hoffmann, I. Kielmann, W. Ulbricht, R. Zana, J. Lang, and C. Tondre, Theory of the kinetics of miceliar equilibria and quantitative interpretation of chemical relaxation studies of micellar solutions of ionic surfactants, J. Phys. Chem., 80, 905-922 (1976). (49) E. A. G. Aniansson and S. N. Wall, On the kinetics of step-wise micelie association,J. Phys. Chem., 78, 1024-1030 (1974). (50) A. Patist, B. K. Jha, S.-G. Oh, and D. O. Shah, Importance of miceliar relaxation time on detergent properties,J. Surf Der., 2, 317-324 (1999). (51) D. Dhara and D. O. Shah, Stability of sodium dodecyl sulfate micelies in the presence of a range of water-soluble polymers: A pressure-jump study, J. Phys. Chem. B, 105, 7133-7138 (2001). (52) D.M. Bloor and E. Wyn-Jones, Kinetic and equilibrium studies associated with the binding of surface-active agents to macromolecules, Chem. Soc. Farad. Trans. 2, 78, 657-669 (1981). (53) C. R. Behl, G. L. Flynn, T. Kurihara, N. Harper, W. Smith, W. I. Higuchi, N. F. H. Ho, and C. L. Pierson, Hydration and percutaneous absorption. I. Influence of hydration on alkanol permeation through hairless mouse skin,J. Invest. Dermatol., 75, 346-352 (1980). (54) M. Sznitowska, S. Janicki, and A. C. Williams, Intracellular or intercellular localization of the polar pathway of penetration across stratum corneum, J. Pharm. Sci., 87, 1109-1113 (1998). (55) G. K. Menon, D. B. Bommannan, and P.M. Elias, High-frequency sonophoresis: Permeation pathways and structural basis for enhanced permeability, Skin Pharmacol., 7, 130-139 (1994). (56) G. K. Menon and P.M. Elias, Morphological basis for a pore-pathway in mammalian stratum cor- neum, Skin Pharmacol., 10, 235-246 (1997). (57) A. C. Williams, P. A. Cornwell, and B. W. Barry, On the non-Gaussian distribution of human skin permeabilities, Int. J. Pharm., 86, 69-77 (1992).
j. Cosmet. sci., 54, 47-52 (January/February 2003) Determination of methylparaben in o/w emulsions by solid-phase extraction and hioh-performance liquid chromatooraphy NONGNUCH PONGCHAROENKIAT, ARUNEE WITTAYANUKULLUK, and STANLEY L. HEM, Department of Indratrial and Physical Pharmacy, Purdue University, W. Lafayette, IN 47907. Accepted for publication April 29, 2002. Synopsis A simple, fast, and accurate solid-phase extraction (SPE) using a 1-cc Oasis HLB cartridge for sample clean-up followed by an HPLC analysis for the assay of methylparaben (MP) in an o/w emulsion is described. One milliliter of methanol followed by 1 ml of 10% methanol in water was used to activate the cartridge sorbent. The sample was loaded into the cartridge and MP was then separated from oil-soluble excipients by washing the cartridge with 1 ml of 10% CH3CN in water. MP was finally eluted from the cartridge with mobile phase, acetonitrile and water (60:40), and quantified by HPLC analysis on a Nova-pak © C-18 column at 254 nm. INTRODUCTION Methylparaben (MP) is one of the most widely used preservatives in pharmaceutical, cosmetic, and personal care emulsions due to its broad antimicrobial spectrum and relatively low toxicity (1). The determination of MP in these products is required to assure its efficacy. One of the frequently employed assay methods is high-performance liquid chromatography (HPLC) (2-5). Due to the complicated matrices of emulsions and the relatively small concentration of MP present, direct injection of emulsions into the HPLC system may result in lower accuracy. A convenient alternative to direct injection of emulsions into the HPLC system is to dissolve the emulsion in a water-miscible solvent or mixture of solvents. Unfortunately, the wide range of hydrophilic and hy- drophobic components of a cosmetic or pharmaceutical emulsion frequently precludes this approach. Thus, extraction is frequently needed prior to HPLC analysis. Nongnuch Pongcharoenkiat's current address is Government Pharmaceutical Organization, Bangkok 10400, Thailand. Address all correspondence to Stanley L. Hem. 47
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)