48 JOURNAL OF COSMETIC SCIENCE A classical procedure for extracting MP from complicated matrices is liquid-liquid extraction (LLE) (6). However, LLE is time-consuming and requires a large volume of organic solvent to isolate the analyte. On the other hand, solid-phase extraction (SPE) can be performed in a shorter period of time and generates a significantly smaller amount of waste. SPE is based on a principle similar to that of chromatography and may utilize normal- phase, reversed-phase, and ion-exchange adsorbents. Reversed-phase SPE, in which a nonpolar stationary phase, the sorbent, retains the nonpolar compounds including the analyte and allows the polar compounds to pass through, was used in this study. An eluent is then used to extract the retained analyte from the sorbent. Although SPE procedures have been proposed to extract MP, evaporation of the extract in order to remove the organic solvent was still necessary (7,8). The drying process in these procedures may reduce assay accuracy due to the degradation of the MP. The goal of this study was to develop a simple, fast, and accurate SPE technique using a small volume of organic solvent without a drying step in order to separate MP from other components of o/w emulsions. EXPERIMENTAL REAGENTS MP, butylparaben (BP), butylated hydroxytoluene (BHT) (Aldrich, Milwaukee, WI), Miglyol 810 (Huls Aktiengesellschaft, Hillside, NJ), olive oil (ICN Biomedical, Aurora, OH), glycerol (J. T. Baker Inc., Phillipsburg, NJ), methanol, and acetonitrile (HPLC grade, Mallinckrodt, Paris, KY) were obtained commercially. Egg phospholipid was a gift of Pharmacia & Upjohn (Clayton, NC). Doubly distilled water (ddH20) was used throughout the study. PREPARATION OF EMULSIONS A 20% w/w oil-in-water emulsion containing 0.1% MP was prepared according to the procedure reported in a previous study (9). The oil phase was composed of 0.1% w/w MP, 0.2% w/w BHT, and 10% w/w each of Miglyol 810 and olive oil. The aqueous phase consisted of 1.2% w/w egg phospholipid, 2.4% w/w glycerol, and ddH20. The oil phase was prepared by dissolving BHT and MP in a mixture of Miglyol 810 and olive oil using a sonicator (model 1200, Branson Ultrasonic Corporation, Danbury, CT). The aqueous phase was prepared by dispersing egg phospholipid in the mixture of glycerol and ddH20 by use of a constant-speed stirrer (Stedi-Speed, Fisher, Fairlawn, NJ) at 1000 rpm. A pre-emulsion was prepared by adding the oil phase to the aqueous phase and mixing at 1000 rpm for 30 min. Final emulsification was completed by passing the pre-emulsion through a microfluidizer (model 110Y, Microfluidics Corporation, New- ton, MA) at 20,000 psi five times. Nitrogen gas was bubbled continuously during the preparation of the emulsion. The precision and accuracy of the quantification of MP was tested by adding MP to the finished emulsion. Two aqueous stock solutions of MP containing 1.5 mg MP/ml or 2.0 mg MP/ml were prepared. Spiked emulsions were prepared by adding 0.10 ml of the 1.5
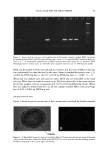
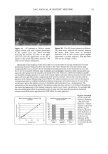
METHYLPARABEN IN O/W EMULSIONS 49 mg MP/ml stock solution or 0.10, 0.15, 0.20, 0.26, or 0.30 ml of the 2.0-mg MP/ml stock solution to 10 ml of the emulsion. The six spiked emulsions ranged from 100.5 % to 103.0% of theory in 0.5% intervals. The spiked emulsions were mixed for 30 minutes with a magnetic stirrer and analyzed in duplicate for MP. A blank emulsion was prepared without MP. ANALYTICAL PROCEDURE The separation of MP from the emulsion was accomplished by solid-phase extraction using a 1-ml Oasis HLB 1-cc (30 mg) cartridge (Waters, Milford, MA). This cartridge was activated by passing 1 ml of methanol through the column by gravity flow to solvate the alkyl chains of the sorbent. The cartridge was then conditioned with 1 ml of 10% methanol in ddH20. A sample (0.1 to 1.0 ml) of emulsion was then added to the cartridge. The MP, as well as unwanted compounds, was retained in the sorbent. The cartridge was then washed with 1 ml of 10% acetonitrile in ddH20 to elute the unwanted compounds. It was assumed that the washing solution, which is weaker than the eluent, minimally elutes the MP from the sorbent. MP was finally eluted from the sorbent with 1 ml of acetonitrile:ddH20 (60:40) and collected in a 25-ml volumetric flask. One half milliliter of 0.1% BP in acetonitrile:ddH20 (60:40) was added as an internal standard. The final volume was adjusted to 25 ml with acetonitrile:ddH20 (60:40). The average of two replicates was reported. The concentration of MP in the eluent from the Oasis HLB cartridge was determined in triplicate by HPLC. The mobile phase was acetonitrile:ddH20 (60:40). A 3.9 x 150- mm Nova-pak C-18 reversed-phase column (Waters, Milford, MA) was used. The HPLC system consisted of a 20-pl injector (model 715, Rheodyne, Cotati, CA), pump (model 510 Waters, Milford, MA), automated gradient controller (model 680, Waters, Milford, MA), UV/visible wavelength detector (model 441, Waters, Milford, MA) and integrator (model 3390A, Hewlett-Packard, Wilmington, DE). The flow rate was 1.0 ml/min. UV detection was carried out at 254 nm at 0.05 a.u.f.s. A stock solution of 1 mg MP in the mobile phase was prepared. The stock solution was diluted with additional mobile phase to obtain solutions ranging from 10 to 40 pg MP/ml that were used to prepare the standard curve. BP (20 pg/ml) was incorporated in the standard solutions as an internal standard. The MP content of the eluent from the Oasis HLB cartridge was determined by finding, from the standard curve, the MP concentration that corresponded to the average peak area ratio of MP and BP. RESULTS AND DISCUSSION Figure 1A shows a typical chromatogram obtained when an MP and BP standard solution was analyzed. The resolution factors for MP and BP were 7.5 and 5.2, respec- tively, confirming that the selected HPLC system was appropriate for analysis of MP. The calibration curve, based on the peak area ratios of MP to BP, was constructed by performing linear least-square regression on triplicate analysis of four standard solutions (10, 20, 30, and 40 l•g/ml). The equation of the calibration curve relating the peak-area ratio (y) to the methylparaben concentration (x) in this range was y = 0.0479x + 0.2199, with R 2 = 0.995. The reproducibility of the retention time of MP (1.4 min) and BP (2.5
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)