2002 ANNUAL SCIENTIFIC MEETING 87 SOLVENT POLARITY AND SUNSCREEN PHOTOSTABILITY Craig Bonda The C.P. Hall Company, Bedford, IL Introduction Many sunscreens degrade (lose absorbance) when exposed to the ultraviolet (UV) radiation found in sunlight. To a first order, sunscreen photodecay can be described as an exponential decay function, Q(a)=Ae •, where Q(a) is the absorbance at a given UV radiation dose r, A is the original absorbance, e is the natural base, and k is the rate constant of photodecay. Photodecay can be determined at any wavelength of interest. Typically, in our lab, we measure photodecay at 370 nm in order to isolate the effect of UV radiation on the UVA filter, avobenzone. Ifk = 0 then the sunscreen is photostable. Commonly, for sunscreens that contain avobenzone, k 0. In particularly photolabile formulations, the magnitude ofk can exceed -.25 indeed we've seen it as high as -.47. At that rate, absorbance at 370 nm virtually disappears after a radiation dose equivalent to about 20 MED (1 MED = 21 rnJ/cm2). The phenomenon of photodecay is best understood as a competition between the many pathways a molecule can take between its elevation to an excited state and its return to the ground state. All of the pathways result in the dissipation of excited state energy. Some of the pathways are destructive to the molecule (e.g., fragmentation, some types of isomerization, bimolecular reaction) others are non-destructive (e.g., fluorescence, phosphorescence, some types of isomerization, energy transfer to another molecule). Each pathway is associated with its o•vn rate constant. If non-destructive pathways predominate, then, relatively speaking, the sunscreen will be photostable. Optimizing sunscreen performance depends in part on encouraging a photolablie UV filter such as avobenzone to dissipate its excited state energy through non-destructive pathways. One strategy to accomplish this is to include in the formulation a chemical that will "quench" the excited state energy of the avobenzone. Chemicals commonly used for this purpose are diethylhexyl 2,6-napthalate, octocrylene, and, in Europe, methylbenzylidene camphor. Patent and regulatory considerations often prevent formulators from employing these chemicals in their formulations. Excited state quenching and its opposite phenomenon, sensitization, represent the intermolecular transfer of excited state energy and, for organic chromophores, it relies primarily on charge transfer i.e. the movement of an electron from one molecule to another. I Electron transfer processes are affected by solvent interactions. Therefore, we concluded that an investigation of the relationship between solvent polarity and sunscreen photostability was warranted. Materials and Methods Materials: Vitro-skin, a substrate designed for in vitro testing of sunscreens, was obtained from IMS. Avobenzone was obtained as samples from Roche and Haarmann & Reimer. The other UV filters, solvents, emulsifiers, and other excipients were obtained as samples from well-known conmaercial suppliers of these materials to the cosmetics industry. Equipment: Samples were equilibrated to 25øC in a Lauda Ecoline 019 water bath. Dielectric constant measurements were made with a Scientifica Model 850 Dielectric Constant Meter. A Solar Light Company 16 S Solar Simulator equipped with WG320 and UG-11 filters supplied UV radiation, 290 to 400 nm. Radiation was monitored by a PMA2114 DSC UV-B Detector and dosage controlled by a PMA2100 Controller, also from Solar Light Company. Sunscreen absorbance was measured on a Labsphere UV Transmittance Analyzer 1000S. Methods: For each filter system (precise combination and concentrations of UV filters), oil phases of differing polarity were prepared and their dielectric constants were measured. Sunscreen formulations were prepared from the oil phases as oil-in-water (o/w) emulsions and were closely matched for variables such as pH and oil phase volume. Vitro- skin slides were prepared by using a pipette to spot the pre-hydrated Vitro-skin with 100 gl of sunscreen, then spreading to a uniform film on the slide. The slides were mounted in slide holders and allowed to dry. Following a baseline scan, each slide was subjected to UV radiation in 5 MED increments and a scan made following each increment. The absorbance measurements were transferred to an Excel spreadsheet and the data collected at 370 nm were plotted on a graph. An exponential regression analysis was made using the Excel trend line utility and the equation and coefficient of determination (R-squared value) displayed. The numerical exponential coefficient of e in the equation was taken as the rate constant of photodecay, k. The values of k for each sunscreen (actually, averages of several values) were then plotted against the dielectric constants of their oil phases. A regression analysis of this data was performed on a Texas Instruments TI-85 graphing calculator and a second order polynomial (quadratic) equation then derived.
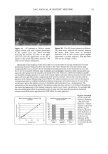
88 JOURNAL OF COSMETIC SCIENCE Results and Discussion The dielectric constants of the individual chemicals in this study, as well as other chemicals commonly found in sunscreens, can be found in Table I. The largest number of formulations tested in this study contained, as UV filters, octyl salicylate (5%), oxybenzone (3%), and avobenzone (2%). The compositions of the formulations' oil phases and the results of the measurements made on these formulations are reported in Table II and, graphically, in Figure I. The results of in vivo studies on four of the formulations are also reported in Table IL The study's early results seemed consistent with a logarithmic relationship between oil phase dielectric constant and rate ofphotodecay (i.e., as the dielectric constant increases, the photodecay rate decreases and the sunscreen becomes more photostable). However, as more data became available, regression analysis determined the best curve fit to be parabolic (i.e. as the dielectric constant increases, the photodecay rate decreases up to a point beyond which it increases.) Other filter systems tested produced confirmatory results. A parabolic relationship between the dielectric constant of the oil phase and the photostability of an avobenzone-containing sunscreen is reminiscent of the theories of Rudolph A. Marcus of the California Institute of Technology. In a series of papers written in the late Fifties and early Sixties, Marcus proposed that the solvent mediates electron transfer reactions and the rate of electron transfer is related to the driving force by a quadratic expression that is descriptive of a parabola? In 1992, Marcus was awarded the Nobel Prize in Chemistry for his contributions. The similarity of our findings to Marcus Theory is perhaps not surprising given the prominent role played by charge transfer in the causation and amelioration of photodecay. Conclusion In many sunscreens, the photodecay rate, k•,d, is related to the polarity of the sunscreen oil phase by the expression, ae: + be + c, where e is the dielectric constant of the oil phase (comprising the solvents, emollients and UV filters), and a, b, and c are empirically derived values for a given filter combination. The expression describes a parabola, the vertex of which identifies the dielectric constant at which photodecay will be minimized and, therefore, sunscreen performance may be optimized. 0 -0.015 -0.03 -0.045 -0.06 -0.075 -0.09 -0.105 -0.12 -0.135 -0.15 Dielectric Constant vs. Photodecay kpd "-.004215s 2 + 0.072748s - 0.33701 Vertex=8.63 R 2 = 0.992 ß Dielectric Constant Figure I Photodecay rate constants of formulations containing, as UV filters, octyl salicylate (5%), oxybenzone (3%), avobenzone (2%) plotted against the dielectric constants of their oil phases. The larger dots represent formulations for which in vivo (five subject, static) SPF studies were completed. Starting from the left, these formulations achieved SPF 17, 20, 21, and 25 respectively.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)