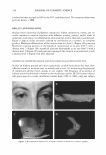
J. Cosmet. Sci., 55, 317-325 Ouly/August 2004) Categorical evaluation of the ocular irritancy of cosmetic and consumer products by human ocular instillation procedures YANG GAO and BRUCE E. KANENGISER, Department of Ophthalmology, Clinical Research Laboratories, Inc., 3 71 Hoes Lane, Piscataway, NJ 08854. Accepted for publication March 19, 2004. Presented at the Annual Scientific Meeting of the Society of Cosmetic Chemists, December 4-5, 2003. Synopsis The assessment of ocular irritation potential is an important part of safety testing for cosmetic and consumer products. The purpose of this investigation was to examine ocular irritancy levels elicited in humans by various categories of a specific class of cosmetic and consumer products that have a potential to enter the eye inadvertently during use. Test materials assessed belonged to one of seven categories, which included liquid makeup, shampoo, baby wash, mascara, eye makeup remover, powder eye shadow, and facial cleanser. These test materials were evaluated by human ocular instillation, followed by examinations, for which subjective perceptions of irritation were recorded, and component areas of ocular tissues were individually examined for inflammation and for the area and density of fluorescein staining patterns at 30 seconds and at 5, 15, 60, and 120 minutes post-instillation. Subjective and objective ocular irritation scores of 410 eyes were analyzed by product classification. Average score levels were determined for subjective responses, inflam mation, and fluorescein staining patterns. This investigation determined that irritation levels of the evalu ated test materials varied markedly with respect to product category, type of ocular irritation, and ocular tissue, demonstrating that these factors are important considerations for the prediction of the ocular irritancy of a test material. INTRODUCTION Ocular irritation testing represents an important step in the safety evaluation of cosmetic and consumer products. Different types of cosmetic and consumer products may irritate ocular tissues at different levels, with various irritancy patterns. A number of studies have been made in which the ocular irritative effects of various compositions of cosmetic products in vitro and in vivo were compared (1,2). There, however, is a relative lack of studies in which human responses have been carefully compared among cosmetic or Address all correspondence to Yang Gao. 317
318 JOURNAL OF COSMETIC SCIENCE consumer products under comparable, controlled conditions. The purpose of this inves tigation was to examine ocular irritancy levels elicited in human subjects by various categories of a specific class of cosmetic and consumer products that have a potential to enter the eye inadvertently during use. MATERIALS AND METHODS SUBJECTS AND MATERIALS The results from 205 human subjects (410 eyes) with normal, non-contact-lens-wearing eyes, ranging in age from 19 to 69 years, were reviewed from 12 ocular instillation studies. All subjects were instructed not to wear any eye makeup for their pre-test qualifying ophthalmic examination. Prior to enrollment, all inclusion/exclusion criteria were verified, an informed consent form was obtained, and a medical history was taken. The test materials assessed, which were provided by multiple clients, belonged to one of seven categories (Table I), which included liquid makeup (9.76%), shampoo (39.02%), baby wash (19.51 %), eye makeup remover (14.63%), mascara (2.44%), powder eye shadow (9.76%), and facial cleanser (4.88%). PROCEDURE OF ACUTE INSTILLATION Each subject was reclined in an automated ophthalmic chair (Isell/Diversatronics, Inc.) at a 60° angle. A 75-100-µl or 30-mg dose of each test material was instilled into the inferior cul de sac, while the lower eyelid was retracted downward. Each test material was instilled in the right or left eye, in rapid sequence. Any excess tearing resulting from the instillation was gently blotted, with a separate tissue used for each eye. Ophthalmic examinations were performed at 30 seconds, 5 minutes, 15 minutes, 60 minutes, 120 minutes, and 24 hours post-instillation. During the course of the study, subjects re mained in a controlled environment to preclude exposure to any external factors that could influence the evaluation of the test material (i.e., rubbing of eyes, smoke, bright lights, etc.). OPHTHALMIC EXAMINATION The ophthalmic evaluation included assessments of subjective reports of ocular symp toms, objective ophthalmic irritation, and ocular surface fluorescein staining. Subjective Products Liquid makeup Shampoo Baby wash Eye makeup remover Mascara Powder eye shadow Facial cleanser Total Table I Distribution of Product Types Preparation Number of eyes Neat 40 10% 160 Neat 80 Neat 60 Neat 10 Neat 40 Neat 20 410 % 9.76 39.02 19.51 14.63 2.44 9.76 4.88 100.00
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)