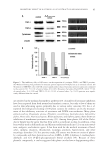
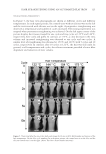
J. Cosmet. Sci., 64, 79–87 (March/April 2013) 79 Modulation of cellular senescence in fi broblasts and dermal papillae cells in vitro JAMES V. GRUBER, PHILIP LUDWIG, and ROBERT HOLTZ, Arch Personal Care, South Plainfi eld, NJ 07080 (J.V.G.,P.L.), BioInnovation Laboratories, Lakewood, CO 80235 (R.H.). Accepted for publication June 7, 2012. Syno psis A hexapeptide (Hexapeptide-11) of structure Phe-Val-Ala-Pro-Phe-Pro (FVAPFP) originally isolated from yeast extracts and later synthesized by solid state synthesis to high purity has demonstrated an ability to in- fl uence the onset of senescence in intrinsically aged fi broblasts, extrinsically aged fi broblasts, and extrinsically aged dermal papillae cells in vitro. The mechanism of senescence control is believed to be related to the pep- tide’s ability to reversibly downregulate ataxia telangiectasia mutated (ATM) and p53 protein expression. The importance of p53 as the gatekeeping protein for monitoring cellular DNA damage is strategic for main- taining cellular health. ATM activates p53 by direct phosphorylation, causing cells to move into senescence which effectively moves them out of reproductive processes. Technologies that can infl uence ATM and p53 expression may offer unique benefi ts for controlling cellular senescence and effectively delaying cellular aging processes. The infl uence on ATM and p53 expression is noted to occur in both cell lines at peptide concentra- tions between 0.1% and 1.0%. The implications of these effects for aging benefi ts for skin and hair is impor- tant as, to date, no known small peptide has been suggested to demonstrate this effect in such a reversible and dose-dependent fashion. INTRODUCTION Cellular replicative senescence accompanies aging and is linked to multiple physical changes in humans ranging from wrinkling and thinning of the skin to hair loss (1). Replicative senescence is a fundamental feature in normal human cells and results par- tially from diminished telomere function at the Hayfl ick limit. The Hayfl ick limit (or Hayfl ick phenomena) is the number of times a normal cell population will divide before it stops, presumably because the telomeres reach a critical length (2,3). When cells reach replicative senescence they stop replicating DNA but continue metabolism (i.e., they continue to make ATP). Radiation and oxidative stress prematurely induces the same phenotypes as replicative senescence prior to the Hayfl ick limit. This process is known as stress-induced premature senescence (SIPS) (4). Address all correspondence to James Gruber at vince.gruber@lonza.com.
JOURNAL OF COSMETIC SCIENCE 80 Fibroblasts are cells that grow in the dermal layer of the skin and are responsible for expression of new collagen and elastin. Dermal papillae cells also grow in the dermis of the skin and are the cells responsible for expression of hair fi bers. Dermal papillae cells from balding and non-balding individuals have recently been grown ex vivo (5). Dermal papillae cells from balding individuals exhibit signature protein markers in- dicating they are experiencing premature senescence. They have been found to express high levels of senescence-associated β-galactosidase (SA-β-Gal) and ataxia telangiecta- sia mutated (ATM) proteins, both being accepted markers for cellular senescence (5). Ultraviolet radiation (UVR)–stressed fi broblasts from aged skin also express high lev- els of these two proteins suggesting senescence plays a strategic role in extrinsic skin aging as well (6). For fi broblasts from aged skin, it has been determined that they express high levels of both ATM protein and SA-β-Gal indicating these cells are ap- proaching or have reached cellular senescence (7). Phenotypically, aging includes effects from the subcellular to macroscopic level. Such signs of aging may be induced or caused by intrinsic factors, e.g., chronological ag- ing, or extrinsic factors, e.g., environmental damage, sunlight, UV, smoke, ozone, pollutants, stress, etc. Visible signs of skin aging include an increase of fi ne lines, wrinkles, large pores, and surface roughness. Recently, it was reported that in mice genetically modifi ed to show accelerated senescence that selective removal of senes- cent cells demonstrated a signifi cant improvement in the aging phenotype of the mice compared against controls in which the senescent cells were not removed (8). The study demonstrates that even though senescent cells make up only a small per- centage of the total cells within the mice, the infl uence of these senescent cells on aging is profound. Biochemical markers for replicative senescence have been identifi ed and include the genes p53 (TP53), Sirtuin1 (SIRT1), ataxia telangiectasia mutated (ATM) and the re- lated ataxia telangiectasia related protein (ATR) (9). In addition, Rad23 (RAD23), p21 (TP21), and p16 (TP16) have also been identifi ed as important markers for cel- lular senescence. ATM protein plays a strategic role in DNA checkpoint response functions linked in part to ATM-directed phosphorylation/activation of p53 and a host of other cellular DNA-damage response proteins (10–12). ATM protein also plays a central role in signaling the presence of DNA double-strand breaks. Loss of ATM protein function is characterized by accelerated telomere loss, genomic instability, progressive neurological degeneration, and premature aging. ATM protein defi ciency and telomere dysfunction likely act together to impair cellular and whole-organism viability. High ATM protein expression is associated with cells in senescence. Changes in expression of ATM protein can also be used as a biomarker to identify senescent cells. Cellular senescence can be observed via various methods. Cellular senescence leads to an increase in SA-β-Gal activity, which can be used as a biomarker to identify senes- cent cells (13). SA-β-Gal is expressed by cells in either intrinsic or stress-induced cellular senescence. Senescent cells can also be noted by changes in the morphology of the cells. It has been reported that extracts from yeast fermentation, in particular, Saccharomyces cerevisiae, have demonstrated wound healing properties (14–19). These physiological effects, which have been variously attributed to increased cellular oxygen consumption
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)