INHIBITORY EFFECT OF GASTRODIA ELATA EXTRACT ON MELANOGENESIS 91 plants residue and boiled for 1 h. The fi ltrate was collected using a 0.2-μm syringe fi lter, combined with the previous fi ltrate sample, and allowed to cool at room temperature. The extracts were stored in the matrix tube and then sterilized using an autoclave. TYROSINASE INHIBITION ASSAY Tyrosinase activity was measured by its DOPA oxidase activity. The tyrosinase inhibition in a cell-free system was tested by mushroom tyrosinase (Sigma, St. Louis, MO). Preincu- bation was conducted with 0.05 ml of 2000 U/ml of mushroom tyrosinase in a 0.1 M phosphate buffer at pH 6.5, followed by incubation in 0.05 M phosphate buffer with the varied concentrations of GE extracts (100, 500, and 1000 μg/ml) and 3 mM L -tyrosine for 10 min at 37°C. The tyrosinase activity was determined by optical density measured at 475 nm using an ELISA microplate reader (Molecular Device Ltd, Wokingham, United Kingdom) and is expressed as a percentage of the control value (100%) an arbutin was used as the reference. CELL CULTURE A pigmented melanoma cell line, HM3KO, established by Ohashi et al. (21) from meta- static melanoma cells of peritoneal fl uids was kindly supplied by Yoko Funasaka (Kobe University School of Medicine, Kobe, Japan). Cells were grown in culture medium con- sisting of Dulbecco’s modifi ed Eagle’s medium with 10% fetal bovine serum (Gibco, Grand Island, NY) and 100 U/ml penicillin–streptomycin in a humidifi ed incubator with 5% CO2 at 37°C. The cells were regularly observed using an inverted microscope and seeded onto 60-mm dishes at a density of 1 × 105 cells per dish. RNA EXTRACTION AND REVERSE TRANSCRIPTION POLYMERASE CHAIN REACTION (RT-PCR) Total RNA was extracted from HM3KO melanoma cells using TRIzol Reagent accord- ing to the manufacturer’s protocol. The RNA samples (2 μg per reaction) were reverse transcribed with reverse transcriptase (Promega, Madison, WI) in the presence of oligo- dT, and the RT product was used for amplifi cation with Taq polymerase. The resulting cDNA was amplifi ed using specifi c primers (Bioneer, Daejeon, Korea) (Table I). Specifi c primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were added as an in- ternal standard for the same reverse transcriptase product. Amplifi cation conditions were 94°C (30 s), 60°C (30 s), and 72°C (40 s) for 35 cycles (tyrosinase), 25 cycles (TRP-1, TRP-2), and 20 cycles (GAPDH), respectively. The PCR products were separated by electrophoresis on 1.2% agarose gels and stained with ethidium bromide. WESTERN BLOT ANALYSIS The HM3KO melanoma cells were seeded onto 60-mm dishes at a density of 1 × 105 cells per dish and cultivated by the method described above. After incubation for 24 h, the medium
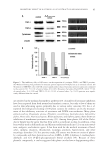
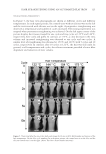
JOURNAL OF COSMETIC SCIENCE 92 was replaced with medium containing 10 μg/ml of GE extract and incubated for an addi- tional 24 h. The medium was then removed, the cells were washed twice with phosphate- buffered saline, and the total protein was extracted using radioimmunoprecipitation assay (RIPA) buffer (Sigma, St Louis, MO) according to the manufacturer’s instructions. The protein content was measured, using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA). An aliquot (30 μg/μl) of protein was fractionated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 10% gel and transferred to a nitrocellu- lose membrane (Schleicher & Schuell, Postfach, Germany). The primary antibodies used were raised against tyrosinase, TRP-1, and TRP-2 (1:500 Sigma). The membranes were blocked with 5% nonfat dry milk and incubated with human tyrosinase, TRP-1, TRP-2, and β-actin monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Band detec- tion was performed using the enhanced chemiluminescence detection system (Amersham Biosciences, Uppsala, Sweden), and band intensity was normalized to the β-actin mea- sured in the same sample. STATISTICAL ANALYSIS Statistical analyses were performed using the Statistical Package for Social Sciences version 11.0 (SPSS Inc., Chicago, IL). Student’s two-tailed t-test was used to evaluate the differences between the study groups. p-values less than 0.05 were considered statistically signifi cant. RESULTS EFFECTS OF GE EXTRACT ON TYROSINASE ACTIVITY To confi rm the inhibitory effect of GE extract, mushroom tyrosinase ELISA was per- formed in vitro. The tyrosinase inhibitory activities of the GE extract were 69.3 ± 7.2% of the control (p 0.05) (Figure 1). EFFECT OF GE EXTRACT ON THE EXPRESSION OF TYROSINASE, TRP-1, AND TRP-2 mRNA As shown in Figure 2, treatment of GE extract on HM3KO melanoma cell signifi cantly reduced tyrosinase mRNA expression compared to the control (43.8 ± 17.4% of control Table I Specifi c Primers for the Human Tyrosinase, TRP-1, TRP-2s, and GAPDH Gene Primer sequences Tyrosinase 5′-TTGGCAGATTGTCTGTAGCC-3′ 5′-AGGCATTGTGCATGCTGCTT-3′ TRP-1 5′-AGAGATGATCGCGAGGTCTG-3′ 5′-CTGTGCCATGTGAGAAAAGC-3′ TRP-2 5′-AGAGATGATCGCGAGGTCTG-3′ 5′-CTGTGCCATGTGAGAAAAGC-3′ GAPDH 5′-GGCCAGCTTTCAGGCAGAGGT-3′ 5′-TGGTGCTTCATGGGCAAAATC-3′
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)