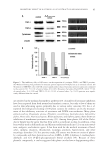
J. Cosmet. Sci., 64, 133–144 (March/April 2013) 133 In vitro melanogenesis inhibitory effects of N-feruloyldopamine S. LEOTY-OKOMBI, S. BONNET, D. RIVAL, V. DEGRAVE, X. LIN, B. VOGELGESANG, and V. ANDRÉ-FREI, BASF Beauty Care Solutions, 69366 Lyon Cedex 07, France (S.L.-O., S.B., D.R., V.D., B.V., V.A.-F.), and BASF Beauty Care Solutions, Stony Brook, NY 11790 (X.L.). Accepted for publication July 23, 2012. The author’s of this article are the employees of BASF. S.L.-O and D.R. are both inventors of U.S. patent US 20110237551 owned by BASF Beauty Care Solutions France S.A.S that protects the use of N-feruloyldopamine in cosmetic preparations. Synopsis Tyrosinase is the rate-limiting enzyme in the melanogenesis process. It remains the most effi cient way to downregulate melanin production and improve unsightly pigmentary disorders. The aim of our investiga- tions was to fi nd a structurally characterized molecule with better effi cacy than existing molecules without cell toxicity. We focused our investigations on compounds that could act as substrate-mimicking inhibitors of tyrosinase and identifi ed N-feruloyldopamine as the best candidate. In vitro, N-feruloyldopamine inhibited human tyrosinase with higher effi cacy than the reference inhibitor arbutin without cell toxicity at least up to 100 μM as measured in cultured normal human epidermal melanocytes (NHEMs). Moreover, the inhibition appeared to be specifi c to mammalian tyrosinases as shown by a very poor inhibition of mushroom tyrosinase, but a signifi cant decrease of total melanin in B16-F10 cells. The antioxidant capacity assessed using DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was comparable to that of vitamin C and fi nally, N-feruloyldopamine exerted a signifi cant inhibition of Pmel17 gene expression when used at 100 μM on cultured NHEM. Taken together, these results suggest that N-feruloyldopamine is a serious candidate for in vivo application as complexion-brightening ingredient. INTRODUCTION Besides increasing concerns about deleterious effects of excessive sun exposure, skin complexion color has become a crucial parameter in the perception of health and beauty (1). Pigmentation disorders that can occur at different moments of a lifetime (pregnancy, old age …), e.g., melasma, freckles, or age spots, can dramatically affect one’s self-confi dence. Medicinal or cosmetic complexion-lightening agents have proved growing popularity. Address all correspondence to Sabrina Leoty-Okombi at sabrina.leoty-okombi@basf.com
JOURNAL OF COSMETIC SCIENCE 134 In mammalian melanocytes, melanin polymers are produced within specifi c lysosome- related organelles called melanosomes. Melanosomes contain three major pigmentary en- zymes: tyrosinase, tyrosinase-related protein-1, and tyrosinase-related protein-2 (dopachrome tautomerase). By catalyzing the two rate-limiting steps in melanogenesis, i.e., the conver- sion of L -tyrosine to L -3,4-dihydroxyphenylalanine (L-DOPA) and its subsequent oxidation to dopaquinone (2,3), tyrosinase plays a critical role in the melanogenesis process (4). Melanosomes undergo a four-step maturation process characterized by different mor- phological stages (I to IV). As observed using transmission electron microscopy, mela- nosomes in stage I appear as round, clear, unpigmented organelles with intralumenal vesicles. Late stage I melanosomes exhibit proteinaceous fi brils that are completely formed in not yet pigmented melanosomes in stage II. The production of internal ma- trix fi bers as well as the maturation of melanosomes from stages I to II depend on the presence of the structural protein Pmel17, also known as gp100 or SILV. Shortly after its delivery from the trans-Golgi network to stage I melanosomes, Pmel17 is cleaved into several fragments, of which some will form the fi brillar matrix of the organelle (5,6). Pmel17 expression, stability, traffi cking, and processing are principally affected by another melanosomal protein called MART-1 (7). The two proteins form a complex, which suggests that MART-1 acts as a chaperone-like structural component for Pmel17 (8).Once the fi brous striations are fully formed in ellipsoidal stage II melanosomes, tyrosinase is transported to stage III melanosomes, which triggers melanin synthesis. Melanin polymers deposit on the fi brils, resulting in their progressive thickening and darkening. Stage IV of so-called mature melanosomes is fi nally described as the stage where internal structures are no longer distinguishable (9). Development of safe yet effective melanogenesis inhibitors is one of the challenges for the dermatological research and cosmetics industry. Despite numerous chemical steps involving several enzymes, transport, and structural proteins, tyrosinase is considered the rate-limiting enzyme of melanogenesis. As a consequence, the majority of com- mercially available skin-lightening ingredients used over the past decades act—at least in vitro—as tyrosinase inhibitors. Kojic acid (1 0), arbutin (11), licorice extract (12), n-butylresorcinol (13) are among the best known members. However, there are some exceptions such as ascorbic acid and niacinamide (14) that were shown to exert their depigmenting effect through signifi cant antioxidant activity and by inhibiting the transfer of melanosome to keratinocytes, respectively. The aim of our research was to develop a structurally characterized tyrosinase inhibitor devoid of cell toxicity. For this, we chose to focus our investigations on compounds hav- ing high structural homology with the natural substrates of human tyrosinase, namely L -tyrosine and L -DOPA and that would thus act as substrate-mimicking inhibitor. This way, we selected N-feruloyldopamine (also referred to hereafter as N-feruloyldopamine), a naturally occurring ferulic acid derivative as the best candidate. To demonstrate the effi cacy of this molecule at inhibiting melanogenesis, its ability to inhibit mushroom and human tyrosinases in vitro as well as melanin production in vitro was assessed. Furthermore, knowing that antioxidant capacities can downregu- late melanin production, its radical-scavenging property was also evaluated. Finally, its capacity to act on melanosome maturation was also assessed by measuring gene expression of three proteins involved in melanosome formation, namely Pmel17, MART-1, and Protein P.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)