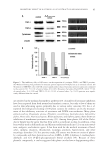
INHIBITORY EFFECTS OF N-FERULOYLDOPAMINE 135 MATERIALS AND METHODS CHEMICALS Ferulic acid, 3-hydroxytyramine, EDCI (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride), L -DOPA, synthetic melanin, 3-methyl-2-benzothiazolinone hydrazone (MBTH), mushroom tyrosinase, dimethyl sulfoxide (DMSO), and 1,1-diphenyl-2- picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France). SYNTHESIS OF N-FERULOYLDOPAMINE N-feruloyldopamine was obtained in a one-step synthesis by means of a peptide cou- pling reaction between ferulic acid and 3-hydroxytyramine in basic medium using EDCI as water soluble coupling agent. Subsequent crystallization affords obtaining N- feruloyldopamine with around 50% yield. The structure of the synthesized compound was confi rmed by nuclear magnetic resonance and mass spectrometry analyses and fur- ther characterized by infrared and high-performance liquid chromatographic (HPLC) analyses (data not shown). The fi nal purity of N-feruloyldopamine was ≥93% as mea- sured using appropriate HPLC method. CELL VIABILITY Cells [normal human epidermal melanocytes (NHEMs) or B16-F10 cells] were seeded at a density of 8000 cells/well in 96-well plates. The B16-F10 cells were cultured in RPMI 1640 medium (Invitrogen, Cergy-Pontoise, France) supplemented with 10% fetal bovine serum (FBS Invitrogen). NHEMs were cultured in keratinocyte serum-free medium (KSFM Invitrogen) sup- plemented with 2% FBS, basic fi broblast growth factor, endothelin-1, α-melanocyte– stimulating hormone (α-MSH), and isoproterenol for 24 h, and then switched to the same medium containing the compounds to test at increasing concentrations and incu- bated for additional 72 h. After incubation, the medium was removed and 0.5 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was then added. After 2 h of incubation, the MTT solution was discarded and fi nally DMSO was added. Absorbance [optical density (OD)] was read at 550 nm using Wallac Victor 2 Spectrophotometer (Perkin Elmer, Turku, Finland). Each condition was tested in sextuplicate (n = 6). HUMAN TYROSINASE INHIBITION ASSAY NHEM were seeded in 24-well plates at a density of 80000 cells per well and grown to confl uence. Inhibitors (N-feruloyldopamine or positive references) were diluted in DMSO and next applied in the culture medium for 24 h at 37°C under 5% CO2. After incuba- tion, the culture medium was removed and human tyrosinase was extracted by lysis of melanocytes by thermal shock. After centrifugation, the supernatants containing tyrosinase were incubated with MBTH and L -DOPA solutions for 30 min before the OD was read at 490 nm. Results are expressed as percentage of tyrosinase activity compared
JOURNAL OF COSMETIC SCIENCE 136 to untreated control (cells grown in the culture medium alone) after normalization to the total protein content beforehand quantifi ed using BCA Assay Kit (Interchim) (15). Kojic acid at 700 μM and arbutin 1 mM were used as positive controls. Each condition was tested in triplicate. MUSHROOM TYROSINASE INHIBITION ASSAY Twenty microliters of inhibitor molecule (N-feruloyldopamine or positive references) diluted in DMSO were incubated for 5 min with 20 μl of a 200 U/ml mushroom tyrosi- nase solution adjusted with 140 μl of phosphate-buffered saline (PBS Sigma-Aldrich). Then, 20 μL of a 10 mM solution of L -DOPA were added, and the OD was read at 490 nm after 10 min of incubation at room temperature. Kojic acid at 70 μM was used as positive control. INHIBITION OF MELANIN SYNTHESIS B16-F10 murine melanoma cells at the 12th passage were seeded in 96-well plates. Cells were grown for 24 h in DMEM medium (Invitrogen) supplemented with 10% FBS (Invitrogen). The compounds to test were then added together with NDP-MSH 10-7M, and cells were incubated for additional 72 h. The media were subsequently removed and cells were rinsed in PBS. After cell lysis, total melanin content was assessed by measuring the absorbance at 405 nm and by referring to a standard curve obtained beforehand using synthetic melanin. Results are expressed as percentage of melanin content compared to untreated control (culture medium + DMSO) after normalization to the total protein content. EVALUATION OF THE ANTIOXIDANT CAPACITY N-feruloyldopamine was dissolved in ethanol at 2X, X representing the fi nal concen- tration in wells. N-feruloyldopamine (100 μl) were dissolved in an ethanolic solution of DPPH at 60 μM. Cysteine at 20 μM was used as positive control. After 30 min of incuba- tion at room temperature, the OD was read at 530 nm. Results are expressed as percent- age of radical-scavenging capacity relative to untreated control. QUANTITATIVE REAL-TIME REVERSE TRANSCRIPTASE PCR NHEM were fi rst cultured at 37°C under 5% of CO2 in KSFM (Invitrogen) supple- mented with 100 μg/ml geneticin (Sigma) and 0.3% normycin (Invitrogen). Cells were then seeded into 24-well plates and cultured for 48 h in KSFM added with the products to test. After washing in PBS, total RNA was extracted using SV 96 Total RNA Isolation System kit (Promega, Charbonnières-lès-Bains, France) according to the manufacturer’s instructions. After quantifi cation at 260 nm, 100 μL of purifi ed total RNA were kept for each sample. Primers used were the following:
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)