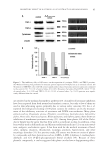
INHIBITORY EFFECT OF GASTRODIA ELATA EXTRACT ON MELANOGENESIS 95 are involved in the melanin biosynthetic pathway (29). A number of tyrosinase inhibitors have been reported from both natural and synthetic sources, but only a few of them are used as skin-whitening agents, primarily due to various safety concerns (30). Lee et al. reported the biological screening of 100 plant extracts for cosmetic use by the measure- ment of the inhibitory activities of tyrosinase and DOPA auto-oxidation. Many plant extracts such as Chaenomeles speciosa, Dryopteris crassirhizoma, Gastrodia elata, Glycyrrhiza glabra, Morus alba, Myristica fragrans, Rheum palmatum, and Sophora japonica have shown an inhibition of mushroom tyrosinase activity (31). Among these plants, GE of the Orchi- daceae family was the agent that has been used as a medicine in Asia. In addition, it has been widely used in folk medicine and Korean traditional medicine (32) as an anticonvul- sant, analgesic, and sedative agent. It has been used for the medical treatment of head- aches, epilepsy, dizziness, rheumatism, neuralgia, paralysis, hypertension, and other neurologic disorders (33). In a previous study, GE extract was shown to consist of pheno- lic compounds and their derivatives such as 4-HBA, 4-HD, 4-hydroxy-3-methoxybenz- aldehyde, and GA [4-(β-d-glucopyranosyl) benzyl alcohol] (34). Because of these phenolic compounds, it can be suggested this plant can also have a depigmenting effect. Figure 3. The inhibitory effect of GE extract on the expression of tyrosinase, TRP-1, and TRP-2 proteins. (A) Western blotting was performed to measure protein expression with β-actin as an internal control. (B) Treatment of HM3KO cells with GE extract signifi cantly reduced tyrosinase protein expression compared to the control (26.1 ± 10.1% of control value, *p 0.05). Moreover, the levels of both TRP-1 (60.6 ± 7.9% of control value, *p 0.05) and TRP-2 (35.8 ± 12.8% of control value, *p 0.05) proteins were signifi - cantly different from the control.
JOURNAL OF COSMETIC SCIENCE 96 Kim et al. (35) reported the extract of GE had inhibitory activity not only against mush- room tyrosinase but also against melanin synthesis in B16 melanoma cells in vitro. Liu et al. reported that 4-HBA, one of the phenolic compounds of GE extract, has an antime- lanogenic effect. This inhibitory effect of 4-HBA is due to the direct inhibition of mela- nosomal tyrosinase activity rather than a decrease in tyrosinase gene expression (36). In our study, GE extract showed about 30% inhibition of mushroom tyrosinase at the con- centration of 100 μg/ml (p 0.05). To examine the tyrosinase inhibitory mechanism of GE extract, we investigated the enzyme involved in melanogenesis and its gene expres- sion. Our results showed that GE extract can reduce the expression of both mRNA and protein of enzymes associated with melanin biosynthesis. The mRNA and protein expres- sion of tyrosinase in the cultured HM3KO melanoma cells was signifi cantly reduced (p 0.05). In addition, examination of melanogenic proteins showed that GE inhibited the expression of key proteins associated with melanin biosynthesis, such as tyrosinase, TRP- 1, and TRP-2 in cultured HM3KO melanoma cells. These results suggest that the in- hibitory effect of GE on melanogenesis is not only by inhibiting tyrosinase catalytic activity but also by inhibiting expression of melanogenic proteins at the transcriptional and translational levels. Further studies are required to elucidate the regulation of tran- scription factor involved in melanogenesis, to check the in vivo effects of GE, and to iden- tify the active component(s) of GE which will be helpful for understanding the mechanism of antimelanogenesis. Moreover, cell study has its limitations so far and we suggest that these results should be replicated in normal melanocytes or human study. ACKNOWLEDGMENT This study was funded by the program of the Kyung Hee University for the young med- ical researchers in 2007 (KHU-20071466). REFERENCES (1) A. Dorner and J. Paweleck, Dopachrome conversion: A possible control point in melanin biosynthesis, J. Invest. Dermatol., 75, 192–195 (1980). (2) J. M. Pawelek and A. M. Körner, The biosynthesis of mammalian melanin, Am. Sci., 70, 136–145 (1982). (3) V. M. Virador, J. Muller, X. Wu, Z. A. Abdel-Malek, Z. X. Yu, V. J. Ferrans, N. Kobayashi, K. Wakamatsu, S. Ito, J. A. Hammer, and V. J. Hearing, Infl uence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes, J. Invest. Dermatol., 117, 1505–1511 (2001). (4) T. Kobayashi, K. Urabe, A. Winder, C. Jiménez-Cervantes, G. Imokawa, T. Brewington, F. Solano, J. C. García-Borrón, and V. J. Hearing, Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis, EMBO J., 13, 5818–5825 (1994). (5) H. Ando, Y. Funasaka, M. Oka, A. Ohashi, M. Furumura, J. Matsunaga, N. Matsunaga, V. J. Hearing, and M. Ichihashi, Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis, J. Lipid Res., 40, 1312–1316 (1999). (6) V. J. Hearing and K. Tsukamoto, Enzymatic control of pigmentation in mammals, FASEB J., 5, 2902– 2909 (1991). (7) G. Battaini, E. Monzani, L. Casella, L. Santagostini, and R. Pagliarin, Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid, J. Biol. Inorg. Chem., 5, 262–268 (2000). (8) K. Maeda and M. Fukuda, Arbutin: Mechanism of its depigmenting action in human melanocyte cul- ture, J. Pharm. Exp. Ther., 276, 765–769 (1996).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)