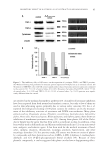
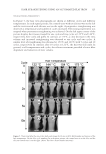
INHIBITORY MECHANISM OF RED GLOBE AMARANTH ON TYROSINASE 103 CIRCULAR DICHROISM MEASUREMENTS Circular dichroism (CD) spectroscopy can be used to estimate the overall conformation of protein molecules. The CD measurements of tyrosinase in the presence and absence of VA were obtained in the range of 190–250 nm using a 2-mm quartz cuvette at a scan speed of 60 nm/min, with the results from three scans averaged for each CD spectrum. Each 1.0 ml mixture contained 0.9 ml tyrosinase (0.1 mg, dissolved in water) and 0.1 ml VA (dissolved in a 10% methanol aqueous solution). The VA/tyrosinase molar ratio was varied (0, 1:1, and 4:1), and the CD spectra were recorded using MOS-450 spectrometer (Bio- Logics Inc., Grenoble, France) at 25°C (19,2 0). ULTRAVIOLET SPECTROSCOPIC MEASUREMENTS Ultraviolet (UV)/visible spectroscopy was used to evaluate whether VA could chelate copper ion of tyrosinase. Spectra at 240–400 nm were measured using the UV-2102 spectrophotometer (Unico Inc., Shanghai, China). The mixture contained 1.9 ml of the samples (10 μg/ml, dissolved in 0.2% methanol) and 0.1 ml of 50 mM phosphate buffer (pH 6.8) with mushroom tyrosinase (100 units/ml). Scans of 1.0 mM CuSO4 were obtained for comparison (21,22). MODEL BUILDING AND MOLECULAR DOCKING The crystal structure of tyrosinase from Bacillus megaterium (TyrBm PDB ID: 3NQ1) (23) was chosen as the protein model in this study. The dicopper and ligands were re- moved from 3NQ1 before the docking computation was performed. The docking algo- rithm was based on the ROSETTALIGAND software (http://www.rosettacommons.org) as previously described (24,25). For each receptor–ligand pair, 500 docking results were generated from the docking calculation. Then, top 10 structures were selected based on the total ROSETTA energy, and they were ranked by the receptor–ligand interaction energy and ligand conformational variation parameters. The fi gures were produced using the PyMOL molecular graphics system (http://www.pymol.org). RESULTS AND DISCUSSION EFFECT OF FRACTIONS FROM RED GLOBE AMARANTH ON TYROSINASE ACTIVITY Table II shows the inhibitory effect of the crude extracts from red globe amaranth fl ower on tyrosinase. Table II shows that the crude extract had a concentration-dependent in- hibitory effect on tyrosinase activity, with an IC50 value of 3.32 mg/ml. These results suggest that the crude extract of red globe amaranth fl ower has potential inhibitory ef- fects on tyrosinase. The inhibitory effects on tyrosinase by additional EA extract fractions are presented in Table III, which shows that the eight fractions (G–N) inhibited tyrosi- nase activity to varying degrees. Overall, the fractions I, K, and N could inhibit tyrosi- nase activity considerably with inhibition rates of 51.05%, 54.37%, and 70.89%,
JOURNAL OF COSMETIC SCIENCE 104 respectively the eluents for these fractions were ether-acetic ether in 8:2, 6:4, and 6:4 ratios, respectively. Further separation yielded 14 fractions, whose inhibitory effects on tyrosinase are shown in Table III. The K fraction generally showed the greatest inhibition of tyrosinase activ- ity. The K6 fraction was especially suitable for inhibiting tyrosinase activity it almost inhibited tyrosinase activity at the concentrations tested. Finally, we successfully identi- fi ed VA from K6 as one of the most effective constituents of the red globe amaranth at depigmenting substrates based on its ability to inhibit tyrosinase activity. Table III Inhibitory Effects of the Fractions on Tyrosinase Activity Fraction Fraction Inhibition rate (%) (Mean±SD) G 40.47 ± 5.46 I 51.05 ± 10.57 I1 44.26 ± 3.42 I2 8.36 ± 1.98 I3 41.89 ± 4.71 I4 20.07 ± 1.70 J 43.57 ± 1.46 K 54.37 ± 9.43 K1 4.81 ± 4.70 K2 48.14 ± 10.34 K3 36.03 ± 10.93 K4 49.04 ± 0.26 K5 58.12 ± 7.36 K6 96.48 ± 2.17 L 31.85 ± 1.37 M 22.43 ± 4.33 N 70.89 ± 7.58 N1 31.60 ± 8.95 N2 36.24 ± 0.19 N3 46.94 ± 3.07 N4 23.30 ± 2.12 Table II Inhibitory Effects of Crude Extracts of the Red Globe Amaranth on Tyrosinase Concentration (mg/ml) Inhibition rate (%) (Mean±SD) 0.35 15.43 ± 1.48 1.75 30.47 ± 1.16 3.50 48.91 ± 0.22 5.25 75.81 ± 1.67
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)